13.6 Using fungi to remediate toxic and recalcitrant wastes
Fungi are quite capable of growing on waxes, paints, leather goods and all forms of textiles, from the finest cotton to the heaviest canvas, and much of this degradative ability results from the activity of lignocellulose-degrading enzymes, particularly the panel of ligninolytic enzymes. As described in Chapter 10, lignin is a polymer of three phenylpropanoid alcohols; so, benzene rings, ether linkages and carbon-carbon bonding predominate in its structure. Lignin is cleaved by an oxidative process that depends on two major groups of enzymes called heme peroxidases and laccases. Together, these enzymes digest away the lignin, which provides the main pigmentation of wood, leaving the white cellulose component for the cellulase enzymes to digest (which is why the the fungi that do this are called ‘white-rots’). The process of catabolic lignin degradation involves:
- cleavage of ether bonds between monomers;
- oxidative cleavage of the propane side chain;
- demethylation;
- benzene ring cleavage to ketoadipic acid which is fed into the tricarboxylic acid cycle as a fatty acid
It is the enzymes that achieve this natural process that have such a high potential for biotechnological applications, especially mycoremediation. For further details you should view:
- Breakdown of polysaccharide: cellulose section CLICK HERE to view the page;
- the section on Lignin degradation CLICK HERE to view the page);
- the section on Digestion of protein CLICK HERE to view the page);
- and processing agricultural, industrial and forest residues in Digestion of lignocellulosic residues in Section 17.22 (CLICK HERE to view the page).
A pest is an organism that is doing what it normally does, but in a place that we consider inappropriate. So a wood-degrading organism growing in the timbers of your house roof is a pest. However, the same organism could be considered a technological marvel if it were recruited to degrade some of our waste products. Overall, on biological and chemical grounds the more advanced fungi, especially the mushroom fungi, are the ideal candidates to degrade the waste vegetation that we produce through our agricultural activities.
On average, world agriculture currently loses 40% of its primary production to pests and diseases, and then throws away more than 70% of what’s left because the crop always represents so little of what is grown. Remember, the ‘crop’ may only be the seeds of the plant that is grown, or even only a portion of the seed, like its oil content. Just imagine how much of the coffee bush ends up in a jar of instant coffee. Typically, 80 to 90% of the total biomass of agricultural production (which amounts, globally, to about 200 billion tonnes per year of organic matter) is discarded as waste and mushroom cultivation is a promising candidate to both reduce environmental pollution and provide food security in the communities that need this most (Bitew & Mandefro, 2018).
Some agricultural wastes are polluted with pesticides. Other agricultural wastes are hazardous because they contain tannins and phenolics (toxic to plants and animals) as residues from extraction of oils, such as cotton, rape, olive, and palm oils, or fruit processing residues, like citrus wastes. These materials are hazardous because they contain compounds chemically similar to the complex phenolic compounds found in wood. Since the fungi can decompose the wood, they can also be used to degrade the environmental pollutants, both in soils and in liquid effluents. The latter including industrial waste water discharges such as those produced by the paper pulp industry, but also wastes contaminated with pesticides, such as chlorinated biphenyls, aromatic hydrocarbons, dieldrin and even the fungicide benomyl.
The advantage is that the fungi do not partially degrade these materials, leaving other possibly dangerous substances behind, rather they completely mineralise the pollutant so that its chemical constituents are returned to the atmosphere and soil as carbon dioxide, ammonia, chlorides and water. Laboratory tests have shown that the oyster mushroom (Pleurotus spp.) is particularly good at this sort of thing. The tests were conducted with pentachlorophenol (PCP), one of the chlorophenols that have been commonly used as disinfectants and preservatives around the world. They do a good job as pesticides but because most environmental microorganisms find them impossible to degrade they persist in the environment and remain toxic for many years. It is illegal to use PCP in most countries today, but it has been the most heavily used pesticide throughout the world. For example, in the United States during the 1980s, approximately 23 million kg y-1 was used, mainly as a wood preservative and since the 1960s approximately 5 million kg was sprayed over vast areas of central China every year as a molluscicide to kill the snails that carry the schistosomiasis parasite. The chemical is very persistent and most of what has been released into the environment is still there. It is highly toxic, uncoupling oxidative phosphorylation by making cell membranes permeable to protons, and thereby dissipating transmembrane proton gradients. It is also cancer inducing and has been declared a priority pollutant for remediation treatment.
The conventional remediation strategy for PCP contaminated land is excavation and incineration or land filling. Such methods are expensive, obviously destructive to the environment and ineffective for anything other than highly localised ‘point source’ pollution. Bioremediation is a very promising alternative, using biological systems for the environmental clean up. The ability of a range of known wood decay or plant litter-decay fungi to remove PCP from a batch culture has been compared by Chiu et al. (1998).
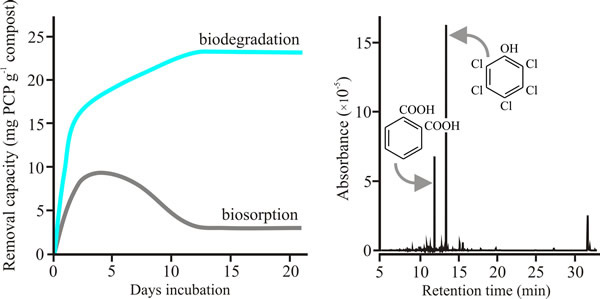
Fungi tested as mycelia were: Armillaria gallica, A. mellea, Ganoderma lucidum, Lentinula edodes, Phanerochaete chrysosporium, Pleurotus pulmonarius, a Polyporus sp., Coprinopsis cinerea and Volvariella volvacea, and the spent mushroom compost from farm beds growing the Oyster mushroom Pleurotus pulmonarius was also tested. All these fungi showed active breakdown and absorption of PCP removal mechanisms, though the tolerance level of the fungus towards PCP did not correlate with its degradative capacity. In a 7 day incubation, A. mellea mycelium showed the highest degradative capacity (13 mg PCP g-1 mycelium dry weight) and Pleurotus pulmonarius mycelium was second-highest with 10 mg PCP g-1 mycelium dry weight; the least effective was Polyporus with 1.5 mg PCP g-1 mycelium dry weight. On the other hand, the Pleurotus spent mushroom compost, harbouring both bacteria and fungi, had a degradative capacity of 19 mg PCP -1 dry weight in only 3 days exposure to PCP (Fig. 5) (Chiu et al., 1998).
 |
|---|
| Fig. 5. Data showing that incubation for a few weeks with spent Oyster Mushroom compost leads to destruction of pentachlorophenol (not just its adsorption)(left hand panel), and that mycelium of Pleurotus pulmonarius dechlorinates pentachlorophenol (PCP) through a catabolic process that involves removal of the chlorine followed by opening of the benzene ring (right hand panel). For the left hand plot, absolute removal capacity of PCP by spent oyster mushroom substrate (that is, the substrate left after the last crop was harvested) was quantified by capillary electrophoresis. The right hand panel shows a mass selective gas chromatography (GC-MS) spectrum of the extract of the fungal biomass of Pleurotus pulmonarius mycelium after two days incubation in a medium containing 25 mg l-1 pentachlorophenol. The most prominent peak (retention time 13.53 min) is PCP, the next most prominent peak at 12.03 min is benzene-1,2-dicarboxylic acid (also called phthalic acid). Other peaks at longer retention times include fatty acids that result from opening the benzene ring and esterification of its straight-chain derivatives. For example, peaks at retention times of 15.73, 31.96 and 32.25 min have been identified as hexadecanoic acid (palmitic acid, C16H32O2) and its derivatives. Modified and redrawn from Chiu et al., 1998. |
Mass-selective gas chromatography (GC-MS) chromatograms revealed only residual PCP peaks in extracts of PCP-treated spent mushroom substrate extracts, a contrast with the mycelial incubations in which a variety of breakdown products were detectable. The spent compost left after oyster mushroom cultivation does two crucial things. It absorbs, immobilises and concentrates PCP so it can be transported away from the contaminated site, and it also digests PCP completely. Mushroom cultivation is a common practice all over the world and the idea that hazardous waste materials could have their pollutants removed and produce a mushroom crop at the same time is exceedingly attractive.
The decontamination of polluted soil and water using fungi is known as mycoremediation (Rhodes, 2014; Barh et al., 2019; Chandra & Enespa, 2019; Dickson et al., 2019; Sánchez, 2020a & b), and there are two approaches: one in which the contaminated material is treated onsite, described as in situ; and ex situ, when the material is physically removed to be treated elsewhere. Removing contaminated soil is the costlier procedure; if the soil can be left and decontaminated in situ, the overall expense is far less. The goal of bioremediation is the full mineralisation of contaminants, that is, their transformation to CO2, H2O, N2, HCl, etc. Heavy metal and radioactive ions can be immobilised by chemical change or accumulated by the fungus and physically removed by harvesting the fungal fruit body.
But the danger in this approach is that the harvested mushrooms must not be used as a food source. Oyster mushrooms can concentrate the metal cadmium (a common industrial contaminant) to such an extent that by eating less than an ounce (dry weight) of the most contaminated samples you would exceed the weekly limit tolerated by humans. Cadmium is so toxic that this situation could pose a public health hazard. There are no worries about conventionally cultivated mushrooms, of course. Indeed, although the cultivation system is known to influence significantly the agronomical parameters and chemical composition of harvested mushrooms (Vieira Jr. et al., 2021), Koutrotsios et al. (2021) have shown that using some wastes as substrates for cultivation, specifically, winery and olive mill wastes, can enhance the nutritional and functional properties of Pleurotus citrinopileatus mushrooms. The point is that if any mushroom is grown on composts that might be mixed with wastes from heavy industry (in remediation programmes, for example), then it should be considered essential to monitor the heavy metal contents before mushrooms are marketed for food, and new biotechnologies are being developed to achieve this (Krakowska et al., 2021).
By far the most promising technique is use of the spent mushroom substrates remaining after harvesting mushroom crops. Ironically, these are often discarded as wastes themselves, but they are clearly able to offer an integrated approach combining soil conditioning with degradation of pollutants as an effective strategy for bioremediation in situ. Spent mushroom farm substrates contain mixed populations of microbes and the synergism that results in such mixtures promises a range of bioremediation approaches to degrade at the molecular level some of the most recalcitrant and toxic organic materials, including oil spills, pesticide spills, and industrial wastes.
Cadmium tolerance of Aspergillus fumigatus has been shown to depend on a gene that encodes a cadmium efflux pump together with extracellular siderophore production. The natural function of the ‘cadmium efflux pump’ could be to protect the hyphae from iron overload by acting as a ferrous iron [iron(II)] pump. This sort of genomics enables understanding of the processes in enough detail to raise the prospects of strain improvement strategies that use gene editing to construct fungal strains specifically for use in biosorption or biomining processes or to prevent accumulation of toxic metals in crops (Kurucz et al., 2018). In Lentinula edodes, possible candidate genes involved in the cadmium accumulation included membrane transporters that respond to chemiosmotic gradients, heat shock proteins, and laccase (Yu et al., 2020).
Petroleum-degrading bacteria can be used for bioremediation of soil contaminated with petroleum sludge, which is the oily sludge generated in refineries when inlet and outlet tanks are cleaned. Disposal of the sludge causes environmental issues as well as human health concerns, but bacterial remediation of contaminated soil can restore its ability to support healthy growth of crop plants (Varma et al., 2017). Successful, and rapid, degradation rates of Total Petroleum Hydrocarbon (TPH) has also been demonstrated by mixed populations of petroleum-degrading bacteria with white-rot fungi grown in solid-state fermentation (see Section 17.21). Enzymes secreted by the white-rot fungus (laccases; see Section 10.7), acting together with the bacteria, degraded the petroleum hydrocarbons in the contaminated soil (Liu et al., 2017), and this includes non-petroleum oil ‘spills/pollutants’ such as palm oil mill effluents (Subowo, 2019; Naranjo-Briceño et al., 2019 ).
Petroleum oil spill is no longer the most dramatically-damaging form of pollution of the natural environment due to human activity; plastics have now reached the number one spot. Plastics have become an essential part of modern life; recent estimates suggest that in 2016, world plastics production totaled around 335 million metric tons. The phrase ‘plastic materials’ covers a range of polymers, including polyvinylchloride (PVC), polyurethanes, polystyrene, polyamides and polyesters with a range of properties and susceptibility to degradation (Sabev et al., 2006; Shah et al., 2008; Sánchez, 2020a & b).
Generally low production costs have led to plastics being used in a vast range of applications; as you can see from a casual glance around your surroundings, from flooring materials, heat insulation, shoe soles, cable sheaths, pipework, packaging, food containers, furnishings, electronic devices and a host of other products that have become essential to modern life. It has been estimated that a total of 8,300 million metric tons of plastics have been produced in the world during the past 65 years; and that, as of 2015, approximately 6,300 million metric tons of plastic waste had been generated, around 9% of which had been recycled, 12% was incinerated, and 79% was accumulated in landfills or just discarded into the natural environment, and between 5 million and 13 million metric tons of plastic end up in the ocean every year (Geyer et al., 2017). It seems that plastic contamination of freshwater fish has a mid-20th century origin. A survey of the digestive tissues of museum specimens of freshwater fish collected from the years 1900-2017 did not detect microplastics in any fish prior to 1950. From mid-century to 2018, microplastic concentrations increased significantly (Hou et al., 2021). All detected particles were fibres, and represented plastic polymers (e.g., polyester) along with mixtures of natural and synthetic textiles. The overall conclusion of these authors was that plastic pollution in common freshwater fish species is increasing and pervasive across individuals and species and is probably related to changes in environmental concentrations of plastic pollutants. This survey is relevant to the wider ocean environment as it has been estimated that about 1% of the world’s rivers account for 80% of global annual emissions of plastic pollution into the oceans, with small urban rivers being among the most polluting (Meijer et al., 2021). Plastic pollution is widespread throughout the marine environment because ocean basins are connected by submarine passages and channels, sometimes referred to as ‘gateways’ (Chiarella & Hernández-Molina, 2021).
The use of plastics has increased twenty fold since 1964, and it is expected to double again by 2035 (Velis, 2014). The world’s synthetic plastic production is projected to be approximately 1,800 million tons by 2050 (Gallo et al., 2018) and approximately 12,000 million metric tons of plastic waste will be in the environment by that year (Geyer et al., 2017).
Poly-(ethylene terephthalate) (PET) is one of the most abundantly produced synthetic polymers and is accumulating in the environment at a staggering rate as discarded packaging and textiles. Unfortunately, the properties that make PET so useful to us in our daily lives also endow it with an alarming resistance to biodegradation, with the potential of it lasting for centuries in most natural environments. Most applications that employ PET, such as single-use beverage bottles, clothing, packaging, and carpeting employ crystalline PET, which is recalcitrant to catalytic or biological depolymerisation due to the limited accessibility of the ester linkages.
PET can be depolymerised to its
constituents if the ester bonds of the polymer can be cleaved. Doing this
with available chemical techniques is too costly to be a viable recycling
solution. Recently, a newly discovered bacterium isolated from
outside a bottle-recycling facility in Japan, Ideonella sakaiensis,
was shown to exhibit the rare ability to grow on PET as a major carbon and
energy source. When grown on PET, this strain produces two enzymes capable
of hydrolysing PET and the reaction intermediate, mono(2-hydroxyethyl)
terephthalic acid. Both enzymes are required to enzymatically convert PET
efficiently into its two environmentally benign monomers, terephthalic acid
and ethylene glycol; so, yielding the monomers for further plastics
manufacture (Yoshida et al., 2016; Austin et al., 2018;
Jenkins et al., 2019 ).
As long ago as 2003, Barratt et al.
(2003) demonstrated that fungi are the predominant micro-organisms
responsible for degradation of soil-buried polyester polyurethane. More
recently, Álvarez-Barragán et al. (2016) found several fungi able to
use polyester polyurethane or a polyether polyurethane varnish as the only
carbon source and found that the fungi were also able to degrade solid
polyester polyurethane foams. FTIR-spectroscopy and GC-MS was used to show
the hydrolysis of ester and urethane bonds in the polyurethane substrates.
Khan et al. (2017) isolated a strain of Aspergillus tubingensis
that was capable of degrading polyurethane from the soil of a general city
waste disposal site in Islamabad, Pakistan. Kemona & Piotrowska (2020) state
that biological degradation of polyurethanes can be applied in
bioremediation of both contaminated water and soil. Between them, these studies have
isolated strains from 12 different species of fungi that can significantly
degrade polyurethanes, demonstrating that there is no difficulty in
isolating efficient polyurethane-degrading fungi from nature, and that the
mechanisms they use to degrade the polymer are accessible to study and could
provide the basis for the development of biotechnological processes for
polyurethane biodegradation and recycling.
A different approach is to study the
degradation rates between different polymers to find biodegradable polymers
that have the potential to be compostable. Al Hosni et al. (2019)
studied biodegradation of the four polymers polycaprolactone (PCL),
polyhydroxybutyrate (PHB), polylactic acid (PLA) and poly(1,4 butylene)
succinate (PBS) in soil and compost over ten months at 25°C, 37°C and 50°C.
PCL showed the fastest degradation rate under all conditions and was
completely degraded after 91 days when buried in compost and incubated at
50 °C. Fungi growing on the polymer surfaces were identified by sequence
analysis. Aspergillus fumigatus was the most commonly found at 25°C
and 37°C, while Thermomyces lanuginosus was abundant at 50 °C, and
could degrade PCL over a range of soil conditions.
For some recalcitrant plastics,
such as PVC, although the polymer itself is highly resistant to
degradation, it is the plasticisers (organic acids blended into the material
to increase flexibility of the product) that are often susceptible to
enzymatic microbial attack; as in, for example plasticised PVC.
Consequently, broad spectrum biocides are often incorporated into polymer
blends to inhibit fungal and bacterial growth and so extend the lifetime of
the final product; which, of course, only increases the adverse
environmental impact when the plastic is discarded.
Phthallates are plasticisers, primarily
used as additives in plastics like polyvinyl chloride (PVC) polymers to make
them more flexible. Because they are not chemically bound, phthalates are
easily released from plastic articles, through direct release, leaching, and
abrasion, and phthalate esters are one of the most frequently detected
persistent organic pollutants in the environment (Gao & Wen, 2015). In
laboratory animal studies, some phthalates have been associated with
developmental and reproductive toxicity and they are generally considered to
be toxins that interfere with endocrine systems in mammals (Hauser &
Calafat, 2005). Di-2-ethylhexyl phthalate (DEHP) is the most commonly used
phthalate plasticiser.
Ahuactzin-Pérez et al. (2018)
discovered that Pleurotus ostreatus degrades and uses (as carbon and
energy source) high concentrations of DEHP, and Fusarium culmorum has
been shown to produce a range of esterase enzymes when challenged with DEHP
(Ferrer-Parra et al. 2018; Portillo-Ojeda et al., 2018;
Ocaña-Romo et al., 2018; González-Márquez et al., 2019a; 2020). The
normal function of many of these esterases is to degrade cutin; that is,
they are cutinases, responsible for
catalysing the cleavage of ester bonds in cutin and other esters. Cutin is a waxy polymer component of the
plant cuticle that covers the aerial surfaces of plants. Authentic apple
cutin will induce cutinolytic esterases when
F. culmorum
is grown in submerged fermentation. Indeed, the
fungus can use apple cutin its sole carbon source and
biomass, protein content and enzymatic activity increased in parallel with
increased cutin concentration in the media
González-Márquez et al., 2019b).
The enzymes would be useful in
several biotechnological areas: as biocatalysts in the food industry; in
detergents; and in bioremediation,
Lack of degradability and growing land and marine pollution problems have led to mounting concern about plastics. With the excessive use of plastics and increasing pressure being placed on capacities available for plastic waste disposal, there is an obvious need for biodegradable plastics, and biodegradation of the plastics that have already been discarded. Despite recycling efforts, most of these materials are discarded into landfill sites or into the environment where they accumulate. Many plastics that contain a carbon-carbon backbone (homopolymers) such as PVC and polystyrene are recalcitrant and resistant to chemical or microbial degradation, whereas others that contain other elements in the backbone (for example nitrogen or oxygen) and are heteropolymers, such as polyurethanes, are highly susceptible to enzymatic microbial degradation. So much so that this can severely damage or limit the useful life of many products made from synthetic polymers, causing major industrial and some medical problems.
As we have just shown, common ascomycete and basidiomycete fungi can produce enzyme systems enabling them to use such pollutants, and the solid polymers themselves, for growth, they provide an opportunity for bioremediation of plastic waste. In Chapter 10 we have shown that the capacity of basidiomycetes to degrade the complex structure of lignocellulose is due to their ability to secrete all the extracellular enzyme systems necessary to degrade biomass. These enzymes have a high potential for biotechnological applications including mycoremediation (Barh et al., 2019; Sánchez, 2020). Recent genomic studies of basidiomycetes have provided valuable information about the variety of enzymes they make available (Peralta et al., 2017); for example, Pleurotus ostreatus degrades lignin efficiently, grows well in both liquid and solid fermentation systems, and is an ideal candidate for genome engineering into a plastic-eating Oyster mushroom. We should all hope that ‘synthetic biology’ (Osbourn et al., 2012) can fulfill its promise and be used to re-design existing biological systems for this essential mycoremediation function. Thankfully, the importance of fungi and of mycology for continued world development and improvement is beginning to be realised (Lange et al., 2012).
Updated September, 2021
