10.2 Breakdown of polysaccharide: cellulose
Polysaccharides are polymers of monosaccharides in which the constituent sugars are connected with glycosidic bonds. Because of the number and variety of available sugars and the diversity of bonding possibilities between different carbon atoms of the adjacent sugar residues there is a considerable variety of polysaccharides. There is a matching variety of enzymes, hydrolases or glucosidases, capable of hydrolysing this range of glycosidic links. Enzymes responsible for polymer degradation (any polymer, not just polysaccharide) may employ one of two strategies of attack. They may attack randomly, effectively fragmenting the polymer molecule into a number of oligomers, these are the endo-enzymes, or they may approach terminally, digesting away monomers or dimers, the exo-enzymes.
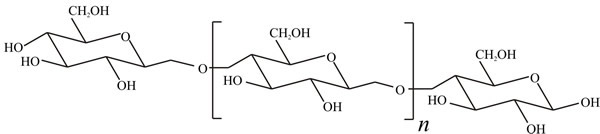
Cellulose is the most abundant organic compound on Earth and accounts for over 50% of organic carbon; about 1011 tons are synthesised each year. It is an unbranched polymer of glucose in which adjacent sugar molecules are joined by β1→4 linkages (Fig. 1); there may be from a few hundred to a few thousand sugar residues in the polymer molecule, corresponding to molecular masses from about 50,000 to approaching 1 million. Breakdown of cellulose is chemically straightforward, but is complicated by its physical form. Mild acid hydrolysis of cellulose releases soluble sugars, but does not go to completion; oligomers of 100-300 glucose residues remain. The fraction which is readily hydrolysed is called amorphous cellulose while that which is resistant to acid is called crystalline cellulose. Since it influences chemical breakdown, the conformation and three-dimensional structure of cellulose must influence cellulolytic enzyme activity.
 |
The cellulolytic enzyme (cellulase) complex of white-rot Basidiomycota like Phanerochaete chrysosporium and Ascomycota like Trichoderma reesei consists of a number of hydrolytic enzymes: endoglucanase, exoglucanase and cellobiase (which is a β-glucosidase) which work synergistically and, in both bacteria and fungi, are organised into an extracellular multienzyme complex called a cellulosome (see next paragraph). Endoglucanase attacks cellulose at random, producing glucose, cellobiose (a disaccharide made up of two glucose molecules) and some cellotriose (a trisaccharide). Exoglucanase attacks from the non-reducing end of the cellulose molecule, removing glucose units; it may also include a cellobiohydrolase activity which produces cellobiose by attacking the non-reducing end of the polymer. Cellobiase is responsible for hydrolysing cellobiose to glucose. Glucose is, therefore, the readily-metabolised end-product of cellulose breakdown by enzymatic hydrolysis.
Cellulosomes were first described in anaerobic cellulolytic bacteria, and are also highly developed in fungi. These enzyme complexes are extracellular molecular machines (‘nanomachines’). In addition to catalytic regions, cellulolytic enzymes contain domains not involved in catalysis, but taking part in substrate binding, multi-enzyme complex formation (so-called ‘docking domains’), or attachment to the cell surface.
Cellulosomes comprise a complex of scaffoldin, which is the structural subunit, and various enzymatic subunits. The intersubunit interactions in these multienzyme complexes are enabled by cohesin and dockerin modules. Cellulosome-producing bacteria have been isolated from many different environments, suggesting that this microbial enzymatic strategy was important and ecologically successful early in evolution. However, the detailed structure of cellulosomes in a given species is variable, and between species there is considerable diversity in the composition and configuration of cellulosomes; species-specific dockerin domains assist enzyme assembly onto cohesin motifs interspersed within variable protein scaffolds (Artzi et al. 2017).
Analogous structures in anaerobic fungi also assemble using non-catalytic dockerin domains and scaffoldin proteins, but these have no similarity to their bacterial counterparts. Cellulosomes in anaerobic fungi contain several enzyme activities not present in their bacterial equivalents. However, some of their catalytic domains are thought to have originated by horizontal gene transfer from bacteria present in the common ancestral environment (like some aspects of secondary metabolism, see Section 17.15). Genomic and proteomic analysis of anaerobic fungi (rumen chytrids) suggest that the fungal cellulosome is an evolutionarily chimeric structure, consisting of an independently evolved fungal complex that co-opted useful activities from bacterial neighbours within the gut microbiome (Haitjema et al., 2017).
Cellulosomes efficiently degrade crystalline cellulose and associated plant cell wall polysaccharides, provide for attachment to the cell surface and their adhesion to the insoluble substrate provides the individual microbial cell that produced them with a competitive advantage in the utilisation of soluble products. Enzymatic degradation of plant biomass has attracted the interest of researchers involved in biofuel production. In particular, because of their efficient organisation and hydrolytic activity, cellulosomes are considered to have commercial potential in this biotechnology. They are the focus of much effort to use the versatile cohesin–dockerin protein assembly mechanism as a standard design principle to engineer ideal ‘designer cellulosomes’ for managing domestic and industrial lignocellulosic wastes (Bayer et al., 2007; Rana & Rana, 2017).
When grown on cellulose, the white-rot fungi like Phanerochaete chrysosporium produce two cellobiose oxidoreductases; a cellobiose: quinone oxidoreductase (CBQ) and cellobiose oxidase (CBO). Cellobiose oxidase is able to oxidise cellobiose to the δ-lactone, which can then be converted to cellobionic acid and then glucose + gluconic acid; cellobiose δ-lactone can also be formed by the enzyme cellobiose: quinone oxidoreductase. Similar cellobiose-oxidising enzymes, capable of utilising a wide variety of electron acceptors, have been detected in many other fungi, though their role is uncertain. These enzymes are probably of most significance in regulating the level of cellobiose and glucose, the accumulation of which can inhibit endoglucanase activity. The role originally ascribed to CBQ was as a link between cellulose and lignin degradation. Cellobiose oxidase also reduces Fe(III) and together with hydrogen peroxide, generates hydroxyl radicals. These radicals can degrade both lignin and cellulose, indicating that cellobiose oxidase has a central role in degradation of wood by wood-decay fungi.
Brown-rot fungi use a rather different initial cellulolytic system to the hydrolytically-based one employed by the white-rots. Brown-rot fungi are able to depolymerise cellulose rapidly and virtually completely. Even cellulose deep within the walls and protected by lignin polymers is prone to attack. The process seems to depend on hydrogen peroxide (H2O2) secreted by the fungus, and iron(II) ions (Fe2+) in the wood oxidising sugar molecules in the polymer, thereby fragmenting it and leaving it open to further attack by hydrolytic enzymes. Interestingly, oxalate crystals that coat so many fungal hyphae may be responsible for reducing the iron(III) ions (Fe3+) normally found in wood to iron(II) ions (Fe2+), so aiding oxidative cleavage of the cellulose.
This ‘oxidoreductive cellulose degrading system’ exists in parallel with the hydrolytic cellulase system already described. The two systems complement one another: copper oxidases attack the highly crystalline region of cellulose, whilst endoglucanases attack amorphous cellulose with cellobiohydrolases that cleave the ends of chains of crystalline cellulose. Oxidative cleavage of cellulose uses Polysaccharide MonoOxygenases (PMOs), Cellobiose DeHydrogenases (CDHs) and members of what is called ‘Auxiliary Activity Family 10’ which is a family of polysaccharide monooxygenases known to cleave chitin and cellulose. Sequences specifying PMOs are widely distributed in the genomes of most ascomycetes and basidiomycetes (white-rot and brown-rot fungi alike) and they boost the efficiency of cellulose degradation by their action on the crystalline part of cellulose which disrupts the tightly packed cellulose chains, making them more accessible for attack by hydrolytic cellulases (Dimarogona et al. 2012).
Updated February, 2020
