10.6 Breakdown of polysaccharide: starch and glycogen
Solid semi-crystalline starch and water-soluble glycogen are two distinct physical states of the same type of storage polysaccharide. Starch, the major reserve polysaccharide of plants, contains glucose polymers with α1→4 glycosidic bonds. Amylose is composed of long unbranched chains, whereas amylopectins (comprising 75-85% of most starches) have branch points formed from α1→6 glycosidic linkages (Fig. 3); these 1→6 bonds form approximately every 20 to 30 glucose units along the chain and the branches are about 30 glucose units long. Starch degrading enzymes include: α-amylases, which are endoamylases acting on 1→4 bonds and bypassing the 1→6 bonds; β-amylases, which are exoamylases producing the disaccharide maltose by splitting alternate 1,4 bonds until they reach a 1,6 branch point (which they cannot bypass); amyloglucosidases (or glucoamylases), which can act on both 1→4 and 1→6 bonds, seem to occur almost exclusively in fungi; debranching enzymes (e.g. pullulanase) which sever 1→6 bonds; α-glucosidases which hydrolyse 1→4 glycosidic linkages in disaccharides and oligosaccharides, producing glucose as the end-product of starch breakdown.
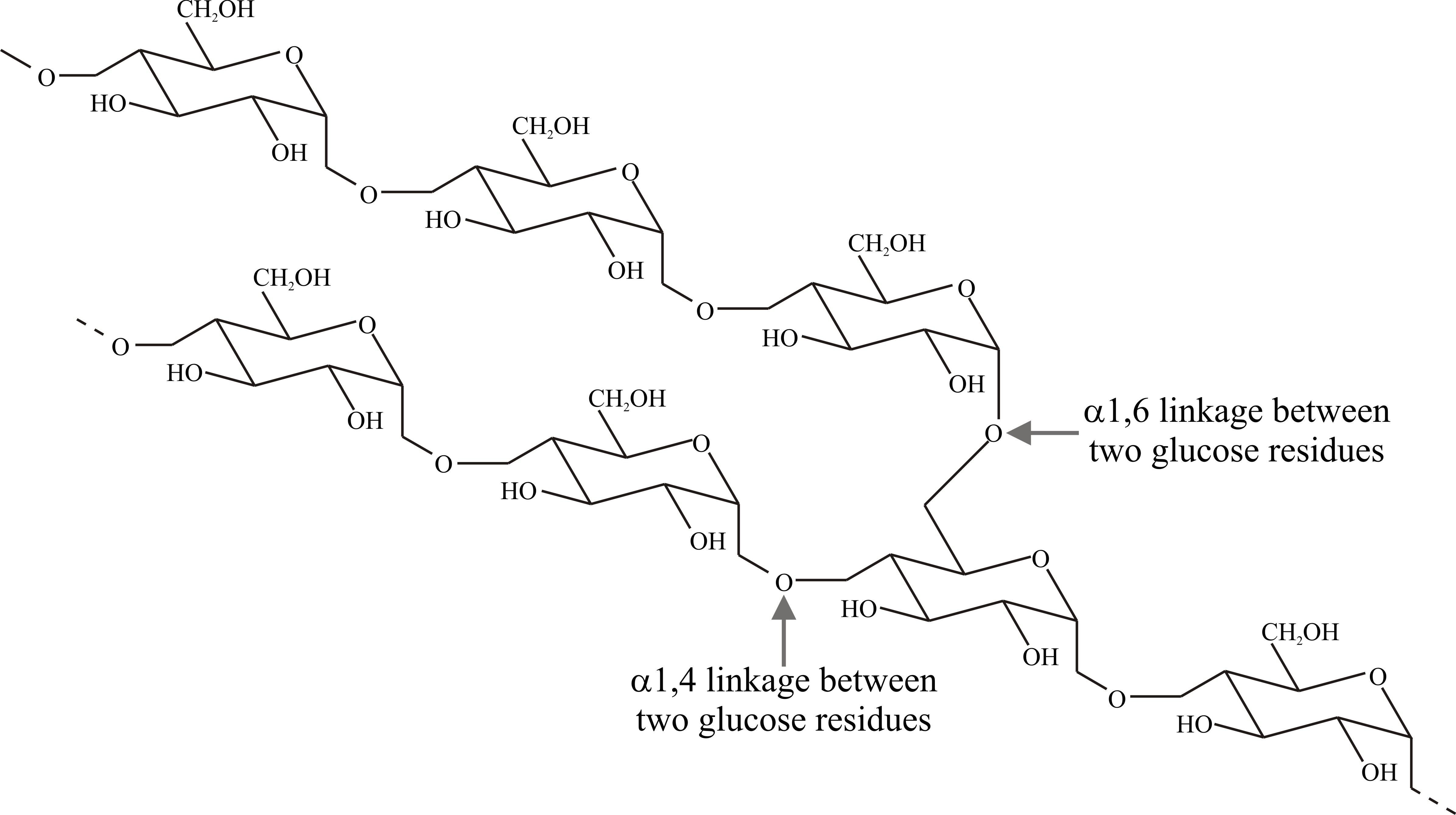 |
Fig. 3. Structural formula of amylopectin. In starch
the 1→6 bonds form approximately every 20 to 30 glucose units along the
chain and the branches are about 30 glucose units long. Modified from
Moore, 1998. |
Glycogen is very similar to starch, being a branched polymer composed of glucose residues linked by α1→4 glycosidic bonds, but branches are shorter and more frequent than they are in starch; about every tenth residue is involved in a branch formed by α1→6 glycosidic bonds in glycogen, and branches are about 13 glucose units long. This is the polysaccharide reserve that’s found in animal tissues, and in the fungi themselves. Most fungi are likely to encounter glycogen in their surroundings, as they are likely to be surrounded by dead and dying fungal cells. Intracellularly, glycogen is degraded by a phosphorylase which releases glucose 1-phosphate for metabolic use (Fig. 4), with the aid of a transferase and α1→6-glucosidase (activities of a single polypeptide) to deal with the branches (plants use a similar phosphorolytic mechanism to mobilise their starch reserves intracellularly using starch phosphorylase to releases glucose 1-phosphate). Extracellularly, glycogen, like starch, is degraded by components of the amylase enzyme complex.
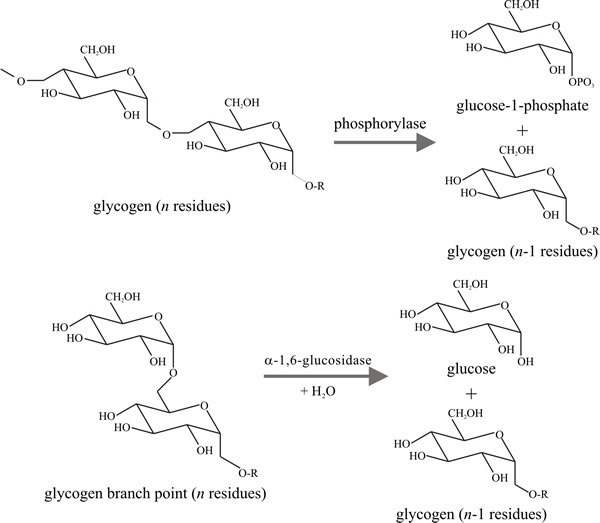 |
Fig. 4. Intracellular degradation of glycogen. The top
panel shows glycogen phosphorylase activity severing one of the 1→4
linkages. The bottom panel shows the ‘debranching activity’ of the
glucosidase hydrolysing a 1→6 linkage. In glycogen, about every tenth
residue is involved in a branch formed by α1→6 glycosidic bonds, and
branches are about 13 glucose units long. Modifed from Moore, 1998.
|
The processes of glycogen synthesis and degradation share similarities among different microorganisms. However, the regulation of that metabolism is different. Glycogen accumulates in Neurospora crassa as carbon and energy reserves to protect against adverse environmental conditions during growth and development (Bertolini et al., 2012). In other organisms, glycogen metabolism seems often to be related to morphogenesis. For example, glycogen is a key storage compound in the rice blast fungus, Magnaporthe oryzae, and the two major enzymes that degrade cellular stores of glycogen regulate plant infection through the NADPH-dependent enzyme trehalose-6-phosphate synthase (Badaruddin et al., 2013). Another example is that in the ectomycorrhizal Périgord Truffle fungus, Tuber melanosporum, transcripts coding for trehalose metabolic enzymes were up-regulated in fruiting bodies, whereas genes involved in mannitol and glycogen metabolism were preferentially expressed in mycelia and ectomycorrhizas respectively (Ceccaroli et al., 2011). The several roles served by glycogen in morphogenesis of sclerotia and fruit bodies of the Ink Cap mushroom Coprinopsis are discussed in Chapter 12.
Updated February, 2020
