17.15 Penicillin and other pharmaceuticals
Citric acid was the first biotechnology product and the techniques developed for production of citric acid by surface cultures of a filamentous fungus in batch mode were applied in the 1940s to production of a much more revolutionary fungal product, the antibiotic penicillin. You have probably heard the story about penicillin being discovered by chance when Alexander Fleming returned from holiday to find that his bacteria had been killed by a contaminating fungus on his Petri dishes. We do not intend to retell that story here because we prefer to emphasise the magnitude of the change in life style which was brought about by the availability of antibiotics. However, the ‘bare-bones’ history of penicillin is as follows:
- 1928, Alexander Fleming, in St. Mary’s Hospital London, discovered his cultures of Staphylococcus aureus were killed by contaminating colonies of Penicillium notatum and is reported to have said some time later: “I must have had an idea that this was of some importance, for I preserved the original culture plate”.
- 1940, Howard Florey and Ernst Chain (of Oxford University), as part of a survey of antibacterial substances produced by micro-organisms showed the chemotherapeutic properties of penicillin.
- 1941, development of penicillin fermentation in USA.
- 1945, Dorothy Hodgkin and Barbara Law (Oxford) used X-ray crystallography to establish the β-lactam structure of penicillin.
- 1956, John Sheehan (USA) produced penicillin by chemical synthesis.
Today we take antibiotics for granted: you have a mildly sore throat so you get an antibiotic; you get a slight injury so you get an antibiotic jab ‘in case of complication’. It’s all become so trivial. So ordinary. But the changes created by the immediate availability of antibiotics were far from trivial. For the first time in human history, antibiotics brought freedom from fear of death from ‘blood poisoning’: a death waiting for everyone for the most trifling of reasons. We can illustrate this with a narrative about one of the first patients to be treated with penicillin.
At the end of December 1940, a man called Albert Alexander was admitted to the Radcliffe Infirmary in Oxford, England. Albert was a 43 years old police officer. He had been injured about a month before admission to hospital, though the record does not say whether the injury was suffered in the line of duty. The injury became infected, the dreaded blood-poisoning or septicaemia. Pathogenic bacteria were spreading through the tissues of his face, head and upper body, growing faster than his immune system could cope with and causing suppurating abscesses all over his face and forehead. His eye became infected and had to be removed on February 3. Then his lungs became infected. He was close to death and was selected for experimental treatment with partially purified penicillin.
On February 12, 1941 he was injected with two hundred milligrams, followed up with one hundred milligram doses at three-hourly intervals. The next day his temperature had returned to normal and he was able to sit up in bed. He continued to improve. But so little penicillin was available that it had to be re-extracted from his urine to be re-injected into his veins. Finally, all supplies were exhausted and his condition worsened rapidly. Despite the early promise of treatment with penicillin there was not enough of the wonder drug to save Albert Alexander. He died on March 15. Killed by an injury that became infected. And the injury that killed this policeman? Albert Alexander had been scratched by the thorn of a garden rose.
More than any other scientific advance of modern times, the penicillin story is able to stir interest and imagination because this one fungal product simply revolutionised medicine. It cannot cure everything but penicillin can be used successfully to treat pneumonia, gangrene, gonorrhoea, septicaemia and osteomyelitis. Diseases that were fatal and widespread, when penicillin first came into use were relegated to medical history. In the 1930s (and before) any injury in which the skin was broken might become infected with soil or air-borne bacteria and some of these might grow beyond the level with which the immune system could deal. When that happened the bacteria were consuming the patient and producing toxins in the blood stream which caused more widespread damage.
An ordinarily-active adult might suffer scratches or minor cuts while gardening, walking, and climbing. Osteomyelitis was an infection of the bone by Staphylococcus which was relatively common in children. Any man who shaved regularly might suffer infection of the inevitable nicks and cuts caused by his razor. This is what killed Lord Caernarvon in 1923. He was a veteran of 19 years in Egypt before he found Tutankhamun’s tomb in 1922. And it was not ‘The Mummy’s Curse’ that finally saw an end to the noble Lord; it was blood poisoning, septicaemia, caused by an infected shaving cut. Less dramatic infections were painful, debilitating, but above all, common.
And women were even more at risk. Birth is a very messy procedure even today and before the ready availability of antibiotics an astonishing proportion of new mothers suffered, and died, from puerperal fever resulting from internal infection. And an equally astonishing number of new-borns were infected during birth with bacteria which were only mildly pathogenic but nevertheless caused blindness, deafness and other life-long disabilities even when the child survived.
Penicillin contributed in a major way to a revolutionary change in medical treatment which in turn changed the human lifestyle to such an extent that diseases which were common causes of death and disability before penicillin are now rarely encountered. This dramatic change to the every-day experience of everyone on the planet is the most remarkable aspect of the discovery and introduction of penicillin.
There are several other remarkable aspects of the penicillin story. Supply is one of them. For the first patient, Albert Alexander, there was little more than one gram available in the whole world and it was not enough to save his life. Today, we produce enough of the totally purified material to give every human on the planet a dose of 5 grams! Development of penicillin production on an industrial scale was a triumph in both scientific and technological terms (Table 9).
Table 9. Penicillin
production: strain development by mutagenesis and strain selection |
||
|---|---|---|
Species |
Strain number and origin |
Concentration of penicillin in culture fluid (mM) |
| Penicillium notatum | Fleming’s original strain |
0.003 – 0.006 |
Sub-strain isolated from the above |
0.035 |
|
| Penicillium chrysogenum | NRRL* 1951 (new species isolated) |
Not recorded |
NRRL 1951.B25 |
0.25 |
|
NRRL 1951.X-1612 |
0.85 |
|
NRRL 1951 Wis Q-176 (ancestor of most present-day penicillin producing strains |
2.5 |
|
| Penicillium chrysogenum | P1 (Panlabs Biologics Inc. San Diego, USA; derived from a strain provided by Toyo Jozo Co. Tokyo, Japan) |
22.9 |
P8 (Panlabs Biologics Inc. San Diego, USA; derived from a strain provided by Toyo Jozo Co. Tokyo, Japan) |
50.0 |
|
| *NRRL = Northern Regional Research Laboratory (now the National Center For Agricultural Utilization Research, the largest of the US Department of Agriculture-Agricultural Research Service research centres) at which the first US National fungal culture collection was opened in 1940. | ||
Penicillin was discovered, apparently accidentally in 1928, by Alexander Fleming. Fleming identified the contaminating fungus as a species of Penicillium and named the unknown inhibitory substance penicillin. Fleming studied the material to some extent during the 1930s, but was unable to purify or stabilise it. He clearly recognised its potential, suggesting that penicillin might have clinical value if it could be produced on a large scale.
Penicillin was later purified by Howard Florey, Ernst Chain, Norman Heatley and other members of a team at Oxford University, which is how the antibiotic became available for experimental use at the Radcliffe Infirmary in Oxford in 1940. The Oxford team developed a purification method using the solvent ether which gave good yields of antibiotic from a meat broth medium on which Fleming’s fungus had been grown. The result was a brown powder which was a remarkably potent antibacterial agent even though still not fully purified. The essence of the production process was a surface fermentation method and the Oxford group used all types of readily-available bottles and dishes, but what proved to be the best of these makeshift containers were hospital bedpans! Later purpose-made ceramic or glass vessels modelled on this utensil were re-named ‘penicillin flasks’.
The British fermentation industry simply lacked the knowledge and expertise to scale-up production of penicillin effectively. But American academic and industrial scientists had experience of growing fungi in deep fermentation submerged culture and were expert in the selection and development of high-yielding strains. Indeed, the crucial American contribution to industrialising penicillin production grew out of the experience of American scientists during the 1930s, like Selman Waksman and Harold Raistrick, in the use of fungi for industrial production of chemicals, especially citric acid.
Transfer of penicillin development to the USA enabled the marvel of war-time antibiotic production to be achieved. By the end of World War 2, penicillin cost less to produce than the packaging in which it was distributed. It made an enormous contribution to the war effort, too. During World War 1, 15% of battle casualties died of infected wounds. When penicillin became widely available in the latter half of World War 2, recovery rates from non-mortal wounds were routinely 94 to 100%; death from infection of the wound was almost zero. The Nobel Prize in Physiology or Medicine 1945 was awarded jointly to Sir Alexander Fleming, Ernst Boris Chain and Sir Howard Walter Florey ‘for the discovery of penicillin and its curative effect in various infectious diseases’ (https://www.nobelprize.org/nobel_prizes/medicine/laureates/1945/).
The microorganism originally chosen for this production process was Penicillium chrysogenum (rather than the P. notatum that Fleming originally isolated). This choice was made on the basis of improved product accumulation in the culture medium (Table 9) and subsequent cycles of mutation and selection considerably improved penicillin yield (Table 9).
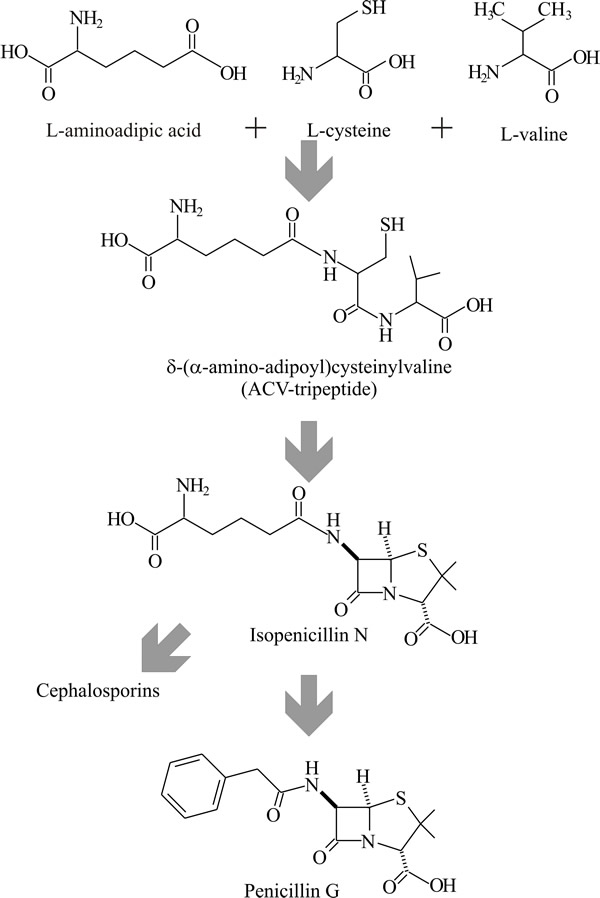
Penicillin is a modified tripeptide made from aminoadipic acid, cysteine and valine (Fig. 29). The principle enzyme involved in penicillin biosynthesis is ACV-synthetase, which forms the tripeptide ACV (δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine) from the three constituent amino acids;
- α-aminoadipate is an intermediate formed during lysine synthesis, it does not accumulate in exponential phase hyphal cells and is not found in proteins,
- cysteine and valine are both found in exponential phase cells and both commonly occur in polypeptides.
 |
|---|
| Fig. 29. Penicillin biosynthesis. Penicillin is a modified tripeptide made from aminoadipic acid, cysteine and valine. ACV-synthetase forms the tripeptide ACV (δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine) from the three constituent amino acids. The β-lactam ring is formed from ACV by isopenicillin-N synthetase; finally, side chains are added or exchanged by acetyl-CoA: isopenicillin-N acyltransferase. |
The second step in penicillin biosynthesis is the formation of the β-lactam ring (as penicillin-N) from ACV by the enzyme isopenicillin-N synthetase; oxygen is involved in the ring closure process but is not incorporated into the molecule. Finally, side chains are added or exchanged by acetyl-CoA: isopenicillin-N acyltransferase in a two-step process:
- release of the isopenicillin-N side chain to yield α-aminoadipate and 6-aminopenicillanic acid (6-APA), then
- addition of a new side chain and release of co-enzyme A.
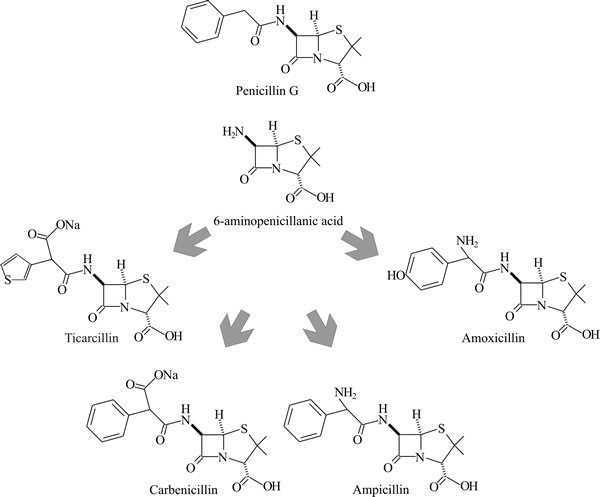
By providing a supply of one particular carboxylic acid, the fungus can be persuaded to produce only one type of molecule featuring the supplied carboxylic acid in its side chain. For example, in the naturally-occurring penicillin-G the side chain = phenoxy acetate; in penicillin-V the side chain = phenylacetate. Semisynthetic penicillins are produced by chemical modification of 6-aminopenicillanic acid (6-APA) (Fig. 30).
 |
|---|
| Fig. 30. Semisynthetic penicillins are produced by chemical modification of 6-aminopenicillanic acid (6-APA). |
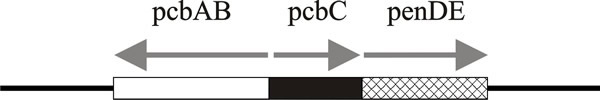
The genes coding for enzymes involved in penicillin biosynthesis are clustered together (Fig. 31). There is a high degree of homology for these genes in all β-lactam producers: gene pcbAB codes for ACV-synthetase, pcbC for isopenicillin N synthetase, and penDE for acetyl-CoA: isopenicillin N-acyltransferase. This is known as a Metabolic Gene Cluster (MGC). MGCs encode various functions in fungi; nutrient acquisition, synthesis and/or degradation of metabolites, etc. As well as encoding the enzymes that perform these anabolic or catabolic processes, MGCs may include integrated regulatory sequences and any mechanisms needed to protect their fungal resident from any toxins they may produce (Slot et al., 2017).
 |
|---|
| Fig. 31. Diagram of the cluster of genes that specify enzymes involved in penicillin biosynthesis and their directions of transcription. Gene pcbAB codes for ACV-synthetase, pcbC for isopenicillin-N synthetase, and penDE for acetyl-CoA: isopenicillin-N acyltransferase. |
This modular, self-contained nature of MGCs contributes to the metabolic and ecological adaptability of fungi and is reminiscent of the developmental subroutines we discussed in 12.14 Classic genetic approaches to study development and the impact of genomic data mining. Being in a cluster enables pathway amplification by gene duplication. This seems to be the basis of the improvements in yield illustrated in Table 17.9. There are 40 to 50 times more copies of the genes in present day industrial strains than in the original Penicillium chrysogenum NRRL 1951 strain. So, it is no surprise that current industrial strains have greatly increased transcription efficiency of genes involved in penicillin synthesis.
The bacterial Streptomyces species also produce β-lactam antibiotics and the high degree of homology between ACV synthetase and isopenicillin-N synthetase produced by Streptomyces and those of filamentous fungi suggests that genes involved in penicillin biosynthesis may have originated in bacteria. It is possible that the genes for the penicillin MGC, having first evolved in bacteria, were subsequently transferred to filamentous fungi by a horizontal gene transfer event, which phylogenetic analysis dates to about 370 million years ago.
Evidence has accumulated suggesting that, through horizontal gene transfer, fungi have been adept at acquiring useful genes from other organisms, particularly bacteria. Horizontal gene transfer is the transmission of genetic material between organisms and across major taxonomic boundaries; that is, from between different species, and even between different domains. The species concept is, of course, difficult to apply to many fungi (see Section 3.9) so it’s probably best to think of horizontal gene transfer as transmission of genes between reproductively isolated genomes.
Note that a consequence of this interpretation is the notion that
‘…fungal species are unique evolutionary units that are separated from one another by boundaries that are ‘porous’ under certain conditions...’ and this ‘…fundamentally affects the population genetics of a species, with potentially profound effects on its overall evolution and biology…’ (Steenkamp et al., 2018).
There is evidence for many horizontal gene transfer events in fungi, that have enhanced both primary and secondary metabolism of many fungi by expanding their sugar, nitrogen, amino acid, nucleobase, and macromolecule metabolism. These transfers have also added significantly to the secretory and metabolite-transporter arrays of fungi, implying that gene transfer has supplemented the basic lifestyle of the fungi concerned (Richards et al., 2011; Slot et al., 2017; see Section 18.8).
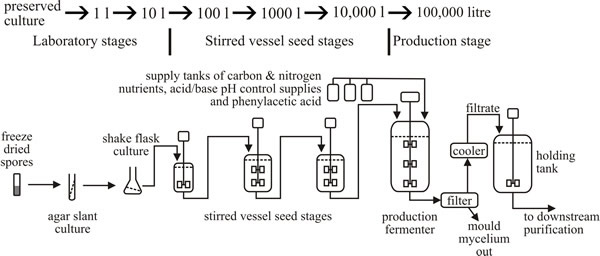
Although original industrial production made use of surface batch cultures, most current production uses fed batch submerged fermentation (Fig. 32). The inoculum for the production stage is amplified through a number of intermediate seed stages. In general each of these inoculum development stages provides a 10% (on a volumetric basis) inoculum for the next stage (top panel in Fig. 32). The production medium may contain glucose, lactose or corn steep liquor (a farm waste product from the wet milling of maize) as carbon source together with inorganic salts. Addition of penicillin precursors (for example, phenylacetic acid) improves yield but these are toxic, and this is the reason for preference for fed-batch fermentation (because the potentially toxic metabolite can be fed at a concentration below the toxic level).
 |
|---|
| Fig. 32. Outline diagram of current penicillin production by fed batch submerged fermentation. Top panel shows the procedure for inoculum development. |
In the production stage a rich medium supports growth for up to 24 h. Nutrient (glucose) becomes depleted and subsequent growth is rate controlled by addition of glucose, the substrate being carefully monitored to ensure it does not reach a concentration which represses penicillin biosynthesis. As the fermentation continues, other nutrients (nitrogen source, sulfur source, penicillin precursor) are depleted (Fig. 33) and additional supplies are provided.
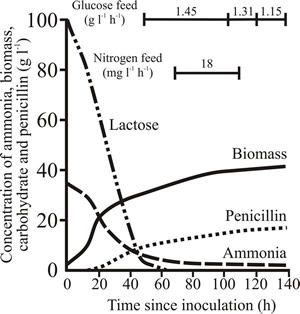 |
Fig. 33. Characteristics of a typical penicillin production run. |
|---|
Typical production costs as a percent of total, are: nutrients, 25%; labour, maintenance, steam, electricity, treatment of waste, etc., 25%; fixed costs, depreciation, taxes, insurances, 28%; separation and purification, 22%. The β-lactams make up 65% of the antibiotics market. Although penicillin can be produced by chemical synthesis, it is much cheaper to produce by fermentation. A 100 m3 fermenter will yield 2.8 metric tonnes of sodium penicillin G in 7.5 days, which will have a ‘factory-gate-value’ of about $70,000. Because penicillin is out of patent it can be produced anywhere in the world and its price varies depending on the number of companies that decide to produce it; but it is always cheap. There are many companies manufacturing antibiotics these days and there are many antibiotics present in the market place. The global antibiotics market was valued at $39.6 billion in 2013 and penicillin represents about 8% of that total (for comparison, tetracyclines make up about 4% of the overall market, erythromycin 7%, streptomycin 1% and chloramphenicol 1% of the market).
Arachidonic acid is another important medicine which is a useful illustration of the use of zygomycete fungi for fermentative production. Arachidonic acid is one of the essential fatty acids required by most mammals. It is a carboxylic acid with a 20-carbon chain and four cis double bonds; the first double bond being located at the sixth carbon from the omega end (chemical name: 5,8,11,14-cis-eicosatetraenoic acid) is a major constituent of the membranes of many animal cells, contributing to their fluidity.
Arachidonic acid has a wide variety of physiological functions in animals and, consequently, is used in many ways in medicine, pharmacology, cosmetics, the food industry and agriculture because in metabolism it is a precursor of:
- prostaglandins, which modulate immune function via the lymphocyte. They are mediators of the vascular phases of inflammation and are potent vasodilators. They increase vascular permeability. Prostacyclin is a vasodilator hormone that works with thromboxane in a homeostasic mechanism in relation to vascular damage.
- Thromboxane; a powerful vasoconstrictor that also increases platelet aggregation; and
- leukotrienes, which mediate inflammation, causing vasoconstriction but increased microvascular permeability.
The physiological pathways to which arachidonic acid contributes in the whole animal are major mechanisms for production of pain and inflammation, and control of homeostatic function.
Some mammals lack the ability to convert linoleic acid into arachidonic acid, or have a very limited capacity to synthesise arachidonic acid, which makes arachidonic acid an essential part of their diet. Plants contain little or no arachidonic acid so these animals are obligate carnivores; the cat family is an example. Humans get their arachidonic acid from both dietary animal sources (meat, eggs, and dairy products) or by synthesis from linoleic acid, but dietary supplementation has significant positive effects. Animal liver, fish oil and egg yolk are well known sources of arachidonic acid but zygomycete fungi of the genus Mortierella are also prominent sources (Table 10) and production levels can be modified by mutations in genes contributing to fatty acid synthesis in M. alpina (Sakuradani et al., 2004).
Table 10. Content of lipid
and of arachidonic acid in Mortierella spp. |
||
|---|---|---|
Fungus |
Fungus lipid as % of dry wt |
Arachidonic acid as % of lipid |
Mortierella alpina |
35 to 40% |
50 to 65% |
Mortierella elongata |
44% |
31% |
Conventional fermentation with Mortierella is a promising production method for easily-purified arachidonic acid, particularly in a two-step fed-batch fermentation process in which impeller speed was reduced after 5 days cultivation from 180 to 40 rpm, and aeration rate adjusted from 0.6 to 1 fermenter volumes per minute. These adjustments decrease physical damage to the mycelia and extend the stationary phase, which is when most fatty acids synthesis occurs. Feeding the batch culture with 3% (w/v) and 2% (w/v) ethanol after 5 and 7 days cultivation, respectively, enhance production further (Jin et al., 2008).
Arachidonic acid has useful clinical effects in lowering cholesterol and triacylglycerols in plasma, with good effects on arteriosclerosis and other cardiovascular diseases. It is present in breast milk and is important in brain and retina development in newborn infants and for these reasons is added to infant feeding ‘formula milk’. Studies have demonstrated that feeding formulas supplemented with arachidonic acid resulted in enhanced growth of infants and provided better developmental outcomes than unsupplemented formulas (Clandinin et al., 2005; Hadley et al., 2016 ).
Updated July, 2019
