17.14 Citric acid biotechnology
Citric acid is a weak 6-carbon organic acid that is a component of the Krebs cycle in which it is generated when the acetyl group from acetyl-CoA is added to the 4-carbon oxaloacetate (see Fig. 9 in Chapter 10; CLICK HERE to view now); so it is a naturally occurring component of metabolism in almost all living things.
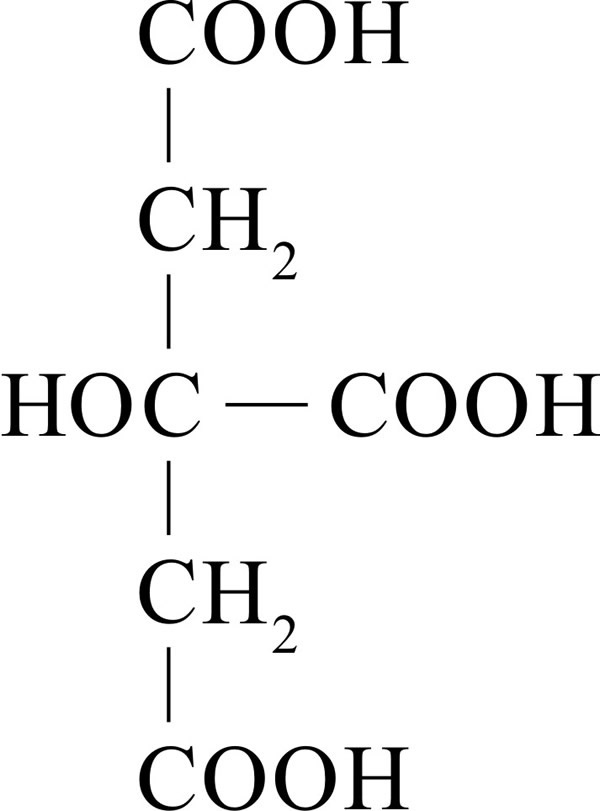
It gets its name from the fact that it is the principal organic acid in citrus fruits where it can account for up to 8% of the fruit’s dry weight. Its chemical structure (Fig. 28) features three carboxylic acid groups and a hydroxyl group, which together confer a variety of properties that make citric acid useful in foods and pharmaceuticals as a pH buffer, acidulant, preservative and/or metal ion chelator.
 |
|---|
| Fig. 28. Structural formula of citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid). |
First isolation of citric acid from lemon juice is credited to Karl William Scheele in 1784 (Show et al., 2015). In 1860, the citric acid industry began, isolating the acid from lemon fruits grown in Italy. This method was used until citrus fruit exports from Italy were disrupted during the 1st World War (1914-1918).
In 1917, James Currie found that the common mould fungus Aspergillus niger could be used to produce citric acid from sugar in a surface fermentation process that became the basis for commercial production employing Aspergillus (Show et al., 2015). Surface culture was the original production method employed for large scale manufacture (introduced in 1919 in Belgium and in 1923 by Pfizer and Co. in the United States).
Although efficient submerged processes have been developed (using the pelleted growth morphology, see Section 17.9; CLICK HERE to view now), surface culture is still employed in some production units as it is simple and has low energy costs. The mycelium is grown as a surface mat in shallow trays of 50 to 100 litre capacity that are made of high purity aluminium or stainless steel.
The main carbohydrate source of the medium may be refined or crude sucrose, cane syrup or beet molasses (diluted to 15% sugars), and the pH is adjusted to 5-7 and the temperature maintained at 28-30°C. Fermentation proceeds over 8-12 days and pH decreases to below 2.0.
After completion of fermentation, the fermented medium is separated from the mycelium by being poured out of the trays. Citric acid is recovered from the fermentation liquor by precipitation with lime (calcium oxide, CaO); the precipitated tricalcium citrate is recovered by filtration and washed several times with water. Treatment of the product cake with sulfuric acid forms insoluble calcium sulfate in a liquor containing citric acid which can be filtered, concentrated and crystallised as citric acid monohydrate.
Submerged fermentation can be performed by batch, continuous and fed batch methods, but batch fermentation is most often used and an aeration system that can maintain a high dissolved oxygen level is essential. Under optimal conditions fermentation is complete in 5 to 10 days. Overall fermentation yields are in the range of 70-75%, and at least 1,600,000 tonnes of citric acid are produced annually (worldwide) by fermentation. China is the largest player in the citric acid market, accounting for 59% of world production in 2015; it also accounted for 74%, and 12% of world exports and consumption, respectively, in 2015 (source: https://ihsmarkit.com/products/citric-acid-chemical-economics-handbook.html).
| For the price of a Coffee & Pumpkin Bread ($5) you can
buy yourself a PDF file of this chapter from 21st Century
Guidebook to Fungi Online. Our PDFs feature an elegantly simple text layout that is easily readable on your mobile or other device, and all hyperlinks are live so you can continue to enjoy the Internet experience. Chapter 17: Whole organism biotechnology, an 86-page PDF file priced at FIVE US$ ($5) Delivered to you by SendOwl Not convinced yet? Download a FREE sample HERE  |
Updated July, 2019
