17.8 Stationary phase
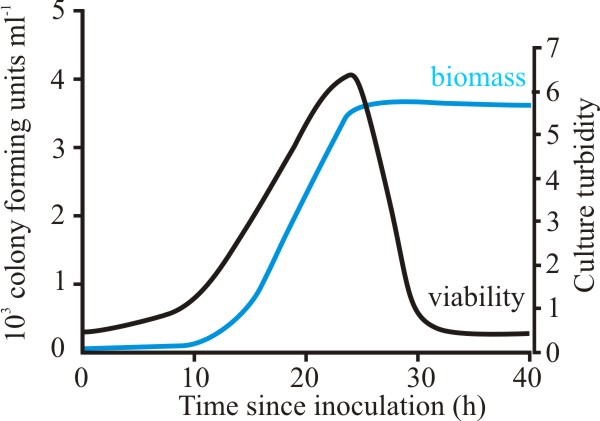
In the stationary phase the biomass does not remain unchanged, rather it becomes senescent. This is associated with a rapid drop in viability as the culture enters stationary phase (Fig. 15).
 |
|---|
| Fig. 15. Growth and viability of Aspergillus fumigatus in batch culture. Growth was followed by using culture turbidity as a measure of biomass, and viability was determined by measuring the colony-forming units per ml. |
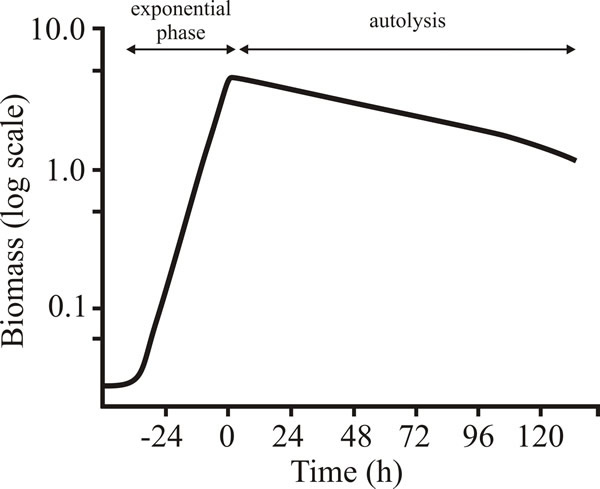
In addition, early stationary phase cultures show signs of autolysis (Fig. 16) following the specific induction of cell features that, in animal systems are diagnostic of programmed cell death (Fig. 17).
 |
|---|
| Fig. 16. Autolysis during stationary phase of batch cultures of Penicillium chrysogenum. The deceleration phase is not apparent in this figure because µmax is maintained until almost all the glucose is used up, and because dry weight is a relatively insensitive method of assessing change in biomass concentration. |
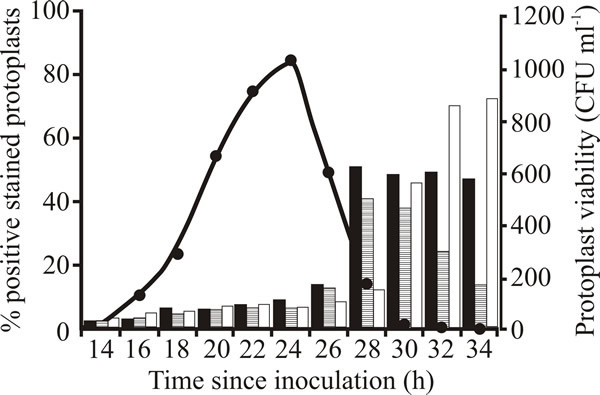
The examples shown in Fig 17 are:
- annexin V-FITC; annexins are a family of calcium-dependent phospholipid-binding proteins. Annexin V preferentially binds to phosphatidylserine (PS) which is predominantly located in the cytosol-facing part of the plasma membrane in healthy cells. An early event in apoptosis is the flipping of PS of the plasma membrane from the inside surface to the outside surface when the membrane begins to become disrupted. Annexin V binds specifically to phosphatidylserine and fluorescently-labelled Annexin V can detect apoptotic cells by detecting PS in the outer membrane leaflet.
- TUNEL staining; terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling (the TUNEL acronym is derived from those words) detects apoptotic cells by labelling DNA strand breaks (‘nicks’) revealing the onset of fragmentation of DNA which is a characteristic of apoptotic cells.
- Propidium Iodide staining (PI): Staining of nuclei with the DNA binding fluorescent (red) dye propidium iodide (PI) also detects fragmented DNA and the intensity of fluorescence correlates with the extent of DNA degradation. Live cells are not permeable to PI, so the stain detects dead cells.
 |
|---|
| Fig. 17. Viability and appearance of apoptotic markers during stationary phase of Aspergillus fumigatus. Protoplasts were prepared from mycelium at various time points and viability (closed circles) was determined. Onset of characteristics diagnostic of programmed cell death was measured as the percentage staining with annexin V-FITC (black bars) to detect plasma membrane disruption, and TUNEL staining (bars with horizontal hatching) and propidium iodide staining (PI; white bars) for fragmented DNA. |
Apoptosis in animals (particularly vertebrates) is a pattern of cell death that maintains cell fragments within an intact plasma membrane until the dying cell is engulfed by a phagocyte. In fact, the distribution of phosphatidylserine on the outside of the cell membrane (referred to above) identifies the dying cells in such animals as targets for phagocytosis. The function of this process is to contain the antigens that might be released by simple cell lysis (necrosis) and protect the animal from autoimmune reactions. This is not an issue in fungi, of course; but the appearance of diagnostic markers of programmed cell death during the stationary phase of a growth curve shows that loss of viability and autolysis in that phase is an organised process. Note our comment in Section 12.15 that in fungal programmed cell death the cell contents released when the sacrificed cells die are specialised to specific functions. The cells are killed as the final step in their growth programme; they don’t just die.
During autolysis, cell wall and macromolecular components in general are degraded; most are simply returned to the medium as the dead cells undergo necrosis, but some of the breakdown products can be scavenging by surviving hyphal cells in the biomass and used for some regrowth. This secondary growth phase is called cryptic growth, this term being coined to remind microbiologists that it is the rule rather than the exception that even during autolysis there is a small (hidden) number of cells that are still growing.
Updated July, 2019
