17.18 The Quorn fermentation and evolution in fermenters
In the late 1950s, forecasters predicted a worldwide shortage of protein-rich foods within 30 years (that is, by the 1980s). It was hoped that single cell protein (SCP), particularly microbes, could provide a means of solving the anticipated world food shortage by industrial production of cheap protein alternatives to meat protein, potentially using wastes as substrates. Fungal SCP must compete with established animal feeds like soya meal, which is a product of conventional farming so a novel microbial protein that requires costly research and development and/or unusual and expensive production facilities will not to be able to compete. The basic requirements for a protein produced for human consumption, as opposed to being a feed material for farm animals, are that it should be:
- cheap to manufacture;
- able to be put into large scale production;
- have a high protein content including essential amino acids.
In 1964, the Rank Hovis McDougall (RHM) Research Centre set out to develop a way of converting starch, which is a waste product from cereal processing, into a protein-rich food. RHM decided to produce its new food from a filamentous fungus because:
- of the long history of man using fungi as food;
- it is relatively easy to harvest fungal mycelia from culture broths;
- it is possible to formulate food products from filamentous fungi which have the appropriate smell, taste and texture, that is the sensory or organoleptic properties, of an acceptable food. The market virtues of the material centre on its filamentous structure which enables it to simulate the fibrous nature of meat. Coupled with the inherent nutritional value of fungal biomass, this permits the product to be sold as a low-fat, low-calorie, cholesterol-free health food. Initially, it was deliberately not compared to meat in any way but sales increased dramatically when marketed as a healthy meat substitute (Table 12) that can be efficiently produced (Table 13).
Table 12. Comparison of Quorn
mycoprotein with beef |
||
|---|---|---|
Nutritional content |
Braising beef |
Quorn |
Protein |
30.9 |
12.2 |
Dietary fibre |
0 |
5.1 |
Cholesterol |
0.08 |
0 |
Fat, total |
11.0 |
2.9 |
Polyunsaturated/saturated fats ratio |
0.1 |
2.5 |
Following an extensive screening programme, a strain of Fusarium
venenatum A3/5, then known as F. graminearum A3/5, was selected
for evaluation (Fig. 34). mycoprotein
is the term coined by
the UK Foods Standards Committee to serve as the generic name for a food product
resulting from the fermentation of Fusarium venenatum. The material was
produced by Marlow Foods Limited, set up in 1984 as a joint venture between RHM,
who had developed the product and Imperial Chemical Industries (ICI) who had
spare fermenter capacity.
Table 13. Comparison of
protein yields per kg glucose used in production |
|
|---|---|
Producer organism |
Protein production (g kg-1) |
Cattle |
14 |
Pigs |
41 |
Chickens |
49 |
F. venenatum |
136 |
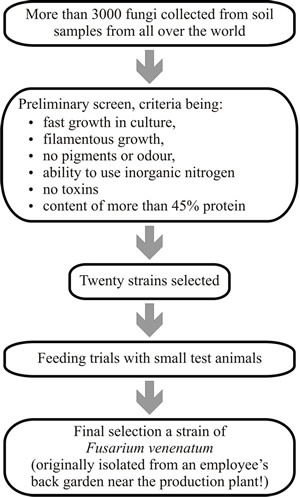 |
Fig. 34. Flowchart of the screening programme that was used to find the fungus that eventually became known as the Quorn fungus. |
|---|
A ten year study (1970-1980) of the product included toxicology testing, involving feeding trials on 11 animal species (including pigs, calves, baboons) that showed no adverse effect on animals or offspring; trials with 2500 human volunteers, which also showed no ill effects or immunological response; and demonstration that the bacteriological load was similar to chicken or fish in storage. Eventually a 2 million word, 26-volume report was submitted to the then Ministry of Agriculture, Fisheries and Food for approval, which was granted, and the product was first sold to the public in January 1985. Additional toxicology testing of Quorn® by AstraZeneca was completed in December 1996 for submission to The United States’ Food and Drug Administration; FDA approval being needed before Quorn® could be sold in the USA. The FDA issued a GRAS notice (GRAS = Generally Recognized As Safe) to Marlow Foods (GRN 91) for mycoprotein as a food ingredient in January 2002, and frozen Quorn® was sold for the first time in the USA in August 2002. No food eaten by humans has been subjected to a more rigorous safety testing than Quorn®!
It is important, though, to appreciate that all this food-safety testing applies to just one rather unusual strain of Fusarium venenatum - the strain designated A3/5 and specifically the isolate designated A3/5/3 which is derived from freeze-dried stock cultures deposited at the International Mycological Institute, UK, in 1969. The genus Fusarium comprises many plant pathogenic species, most of which produce mycotoxins when placed under suitable in vitro growth conditions, particularly nitrogen limitation. These are primarily fumonisins and trichothecenes (a class of sesquiterpenes, Fig. 10.17), but also zearalenone (Fig. 10.21) and gibberellic acids (Fig. 10.18). Certain strains, even including the production strain A3/5/3, of F. venenatum have been shown to produce mycotoxins when grown under severe nitrogen limitation. Fortunately, the fermenter conditions used for mycoprotein production (with excess nutrients present) do not induce mycotoxin production. Nevertheless, samples of Quorn™ mycoprotein are taken regularly from the production line to test for the presence of mycotoxins. Mycotoxins have not been detected in any production run in the long history of mycoprotein production (see discussion and references in Whittaker et al., 2020).
Although mycoprotein™ was originally conceived as a protein-rich food to supplement what was thought to be a declining world supply of conventional foods, by the early 1980s the predicted global shortage of protein-rich foods had not materialised. Consequently, Marlow Foods decided to sell Quorn™ mycoprotein™ as a new healthy food which lacked animal fats and cholesterol, is low in calories and saturated fats. Mycoprotein is also high in dietary fibre (it has more dietary fibre - fungal cell walls - than wholemeal bread), a part of the human diet linked to disease prevention (Colosimo et al., 2021). This dramatic change in marketing policy was justified by a survey in 1989 which showed that almost half the UK population was reducing its intake of red meats, whilst a fifth of young people were vegetarians. In line with current dietary guidelines, mycoprotein is high in protein and fibre, and low in fat, cholesterol, sodium, and sugar. Mycoprotein may help maintain healthy blood cholesterol levels, promote muscle synthesis, control glucose and insulin levels, and increase satiety (Finnigan et al., 2019). A more recent independent systematic review of published nutrition studies has confirmed that “Overall, given growing interest in sustainable proteins and accruing health evidence for mycoprotein, firmer embedment with food-based dietary guidelines is now worthy of consideration” (Derbyshire & Delange, 2021).
In the 1990s, it was decided to market mycoprotein™ as a meat analogue. Today, Quorn™ mycoprotein™ is sold in burgers, sausages and analogues of sliced meats, and as an ingredient in over 50 pre-prepared meals. One of the simplest Quorn foods are the meat-like ‘pieces’, which are mildly flavoured but fully textured and sold for use by the end-user as an ingredient in a wide range of home cooked meals. We have mentioned the food value of Quorn in Section 11.4; here we will concentrate on the biotechnology of its production (Wiebe, 2004; Finnigan, 2011; Finnigan et al., 2019; Whittaker et al., 2020 ).
All the production systems described earlier were considered:
- batch and fed-batch because they have the advantages of being:
- simple system,
- commonly used in industrial fermentations,
- used for manufacture of ‘high market value’ products.
- Though they have the disadvantage of being too expensive when the product is the biomass, because a new fermentation needs to be set up every few days.
- continuous flow cultures have the advantage of providing:
- continuous production of biomass,
- over long periods of time,
- consequently, comparatively cheap.
- The main disadvantages were that the process is technically very difficult, and had not previously been implemented with filamentous fungi on an industrial scale.
A continuous flow culture system was chosen for production of F. venenatum A3/5 biomass because much higher productivities can be achieved in continuous culture than in batch culture. Up to the beginning of 1994, the air-lift fermenter used for mycoprotein™ production was a fermenter originally built by ICI at Billingham to grow the bacterium, Methylophilus methylotrophus for the production of an animal feed (called Pruteen). This 40 m3 fermenter (christened Quorn 1), consisted of an elongated loop about 30 m tall and was operated as a glucose-stat at a dilution rate of 0.17 to 0.20 h-1 capable of producing 1,000 tonnes of Quorn™ mycoprotein™ per annum.
In late 1993, Marlow Foods commissioned a new 155 m3 air-lift fermenter (Quorn 2 built on the site of the old Pruteen fermenter) and this was followed by the construction of Quorn 3, the twin of Quorn 2. This has allowed mycoprotein™ production to be increased to between 10,000 to 14,000 tonnes per annum. The new fermenters are the world’s largest continuous flow culture systems; each cost £37.5M to build, is 50 m tall and weighs over 250 tonnes. By 1997, the two new fermenters had enabled sales of Quorn™ mycoprotein™ to be increased to about £74M per annum.
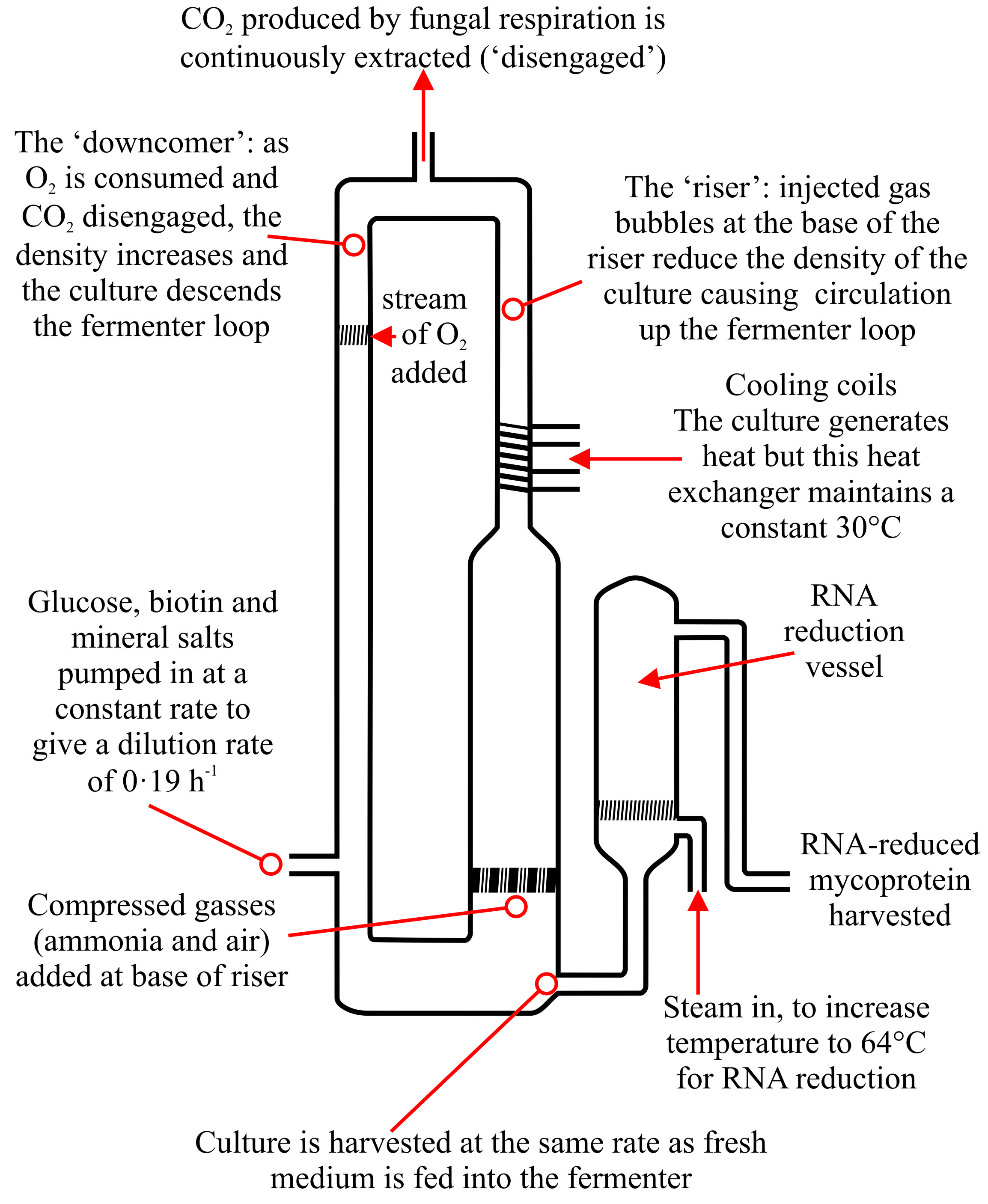 |
Fig. 35. Schematic representation of the Quorn air-lift fermenter used for the production of mycoprotein in continuous flow culture. Adapted from Trinci (1991, 1992, 1994) and Whittaker et al. (2020). |
|---|
Each 155 m3 fermenter is inoculated with 5 1 of a batch culture containing 50 g biomass and continuous flow is initiated 4 days later. In an air-lift fermenter most of the oxygen transfer takes place near the base of the relatively wide riser where sterile air is introduced (Fig. 35) and where the height of the fermenter creates a high hydrostatic pressure. This, together with turbulence (and consequently small bubble size) provides excellent conditions for oxygen transfer from the gaseous to the liquid phase. Thus, the riser contains a two-phase mixture of air and culture flowing together so that air bubbles contribute up to 50% of the volume of the fluid. The rate of transfer of oxygen from the gaseous to the liquid phase decreases as the culture flows to the top of the riser where the gas contains about 10% oxygen.
The low pressure region at the top of the riser causes release of CO2 and the culture then enters the downcomer where, at the bottom, it is directed into the riser and is again charged with air thereby completing the pressure cycle. The culture is maintained at about 30°C by a heat exchanger set into the downcomer. To prevent oxygen-limitation, the downcomer is provided with an oxygen supply. The difference in specific gravity of the relatively aerated culture in the riser and the relatively air-depleted culture in the downcomer (creating a hydrostatic pressure differential) ensures that the growing hyphal filaments circulate continuously around the fermenter loop at a rate of about one circulation every 2 minutes for Quorn 2 and 3, compared to every 6 minutes for Quorn 1.
The nitrogen supply for growth (which is ammonia) is fed into the fermenter with the sterile compressed air, at the base of the riser. The rate of supply of ammonia to the culture is regulated by a pH monitor set to give a culture pH of 6.0. The nutrient solution is fed to the culture to give a dilution rate in the range 0.17 to 0.20 h-1, and is operated as a glucose-stat, that is, glucose is always in excess and the fungus always grows at µmax at a biomass concentration of 10 to 15 g l-1.
The fermenter is manned on a 24 h basis. Tests for the presence of mycotoxins are made every 24 h using a method sensitive to two parts per billion; tests made to date have all been negative, as would be expected for the growth conditions employed (the fungus is growing at µmax whereas toxins are secondary metabolites produced by slow growing or stationary cultures). Product is harvested continuously, and Quorn 2 and 3 each produce an output of 30 tonnes h-1.
The harvested mycelium cannot be used as harvested. If human food contains too much nucleic acid, blood uric acid values rise and the excess accumulates as crystalline deposits in joints and tissues, leading to the disease known as gout, and to kidney stones. This is a peculiarity of human metabolism: in man, uric acid is produced by the breakdown of nucleic acids whereas in other vertebrates the sparingly soluble uric acid is converted to the highly soluble acid allantoin.
Because of this, the World Health Organisation (WHO) laid down a recommendation for human ingestion of RNA from Single Cell Protein (SCP) sources which, for adults, was defined as 2 g RNA per day, with total nucleic acid ingestion from all sources not exceeding 4 g per day. Fusarium venenatum biomass cultured at a specific growth rate of 0.19 h-1 has a RNA content of 8-9% (w/w) which would limit the ingestion of mycoprotein™ to not more than 20 g per day. Consequently, a method was developed to reduce the RNA content of mycoprotein™ whilst minimising loss of protein (though, unfortunately, protein loss is still substantial) and fibrous structure.
In this process, the temperature of the biomass is raised to 68°C for 20 to 30 minutes to stop growth, disrupt ribosomes, and activate endogenous RNAases which break down cellular RNA to nucleotides which diffuse through the hyphal wall into the culture broth. Importantly, RNAases are more heat resistant than proteases, so protein loss is minimised. This method is carried out in the culture broth with no other adjustments, since a pH of 5 to 6 is optimal for RNAase activity. RNA reduction has a substantial economic penalty attached to it, since as well as removing RNA, other cell constituents are inevitably lost during this process, including perhaps up to 30% of the biomass dry weight and a significant amount of protein, but it is essential to reduce the RNA content. After this treatment, the mycoprotein™ contains only 1% (w/w) RNA, similar to that present in animal liver and well within the 2% (w/w) upper limit recommended by WHO.
One of the advantages of using a filamentous fungus rather than a bacterium or yeast for SCP is the comparative ease with which mycelia can be harvested. After RNA reduction, fungal biomass is harvested to give a product which contains about 30% (w/w) total solids. mycoprotein typically contains 44% protein, 18% dietary fibre and only 13% fat (the values for beef are 68%, 0% and 30% respectively). The RNA reduced mycoprotein dough or ‘paste’ is produced at the Quorn Foods Belasis site in Billingham, County Durham, England then transported a few miles to the Quorn Foods Stokesley site in North Yorkshire, for texturing.
Creation of meat-like texture using mycoprotein plus any of a range of gelling and firming agents is a complex process, which is still not fully understood. It clearly depends on the hyphal nature of the mycoprotein, and material properties of protein gels formed by denaturation of other added proteins (egg albumen in original recipes and for vegetarian products; plant proteins like pea and potato proteins in vegan products), and gel-forming agents like alginate, agar, locust bean gum and calcium. The gel is then influenced by freezing. About 50% of the overall texture is achieved after initial freezing with the remainder being developed during about 2 weeks further frozen storage. Beneficial changes during frozen storage are believed to be due to ice crystal growth increasing the bundling of mycoprotein fibres (the mycelium), electrostatic interactions and hydrophobic bonding of aromatic residues exposed during protein denaturation as well as disulphide bonds between cysteine residues. As a result of this processing mycoprotein™ has the same ‘chewiness’ and succulence as meat, but, unlike meat, it locks in colourings and flavourings even when cooked. Quality control checks are carried out at every stage of the process to ensure that the end product is of a consistently high quality.
Although Fusarium venenatum fermentations were originally intended to run indefinitely, in practice, they are terminated 1000 h (about 6 weeks) or less after the onset of continuous flow. Although the shear forces experienced by mycelia in stirred tank (the RHM method) and air-lift (the Marlow Foods method) fermenters differ, the morphology and growth of mycelia of Fusarium venenatum in the two types of fermenter are similar, and highly branched (colonial) mutants (Fig. 36) arise in both systems at approximately the same time after inoculation.
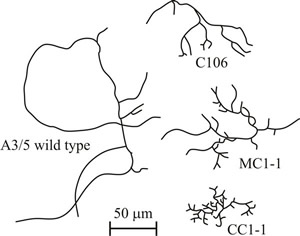 |
Fig. 36. Comparison of the morphology of mycelia of Fusarium venenatum A3/5 and three highly branched (colonial) mutants (C106, CC1-1 and MC1-1), now called ‘c-variants’, that arose spontaneously in experimental laboratory chemostat cultures. |
|---|
Biomass made up of colonial mutants possesses the same chemical composition and is as nutritious as the parental strain, but presence of colonials changes the texture of mycoprotein causing it to become more easily crumbled (friable), so the product standard cannot be maintained. The culture then needs to be ended and a new one started; appearance of colonials causes premature termination of the fermentations and consequent loss of productivity. But note that there are patents that define the use of the friable mycoprotein for vegan analogues of dairy products (‘milk’, ‘cream’, ‘ice-cream’ and spreads similar to butter) because the texture of the colonials mimics the fat globules that make up the authentic dairy products. When grown in plate culture, highly branched mutants form colonies which are much more compact than those of the parental strain and expand in radius much more slowly than the parental strain (this is why they are called colonial mutants). Despite their reduced radial growth rate, in turbidostat or chemostat culture at a high dilution rate, colonial mutants rapidly supplant the parental strain (Fig. 37).
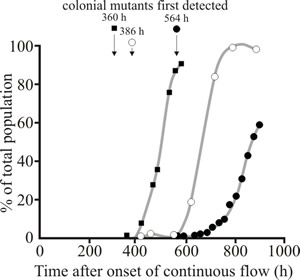 |
Fig. 37. Colonial mutant population (expressed as a percentage of the total population) generated during glucose-limited laboratory chemostat cultures of F. venenatum grown at 0.19 h-1. |
|---|
The colonial mutants appear after about 107 generations (range 99-115). Prevention or delay in the appearance of colonial mutants in mycoprotein fermentations would enhance productivity and decrease the unit cost of the product.
Understanding how this premature termination of the fermentations might be controlled requires an understanding of the evolution of microbial cultures in chemostat culture (Gresham & Hong, 2015). When an organism is grown in a chemostat, the relationship between its specific growth rate (µ) and the concentration of the growth limiting substrate (S) is described by the Monod equation we quoted earlier:
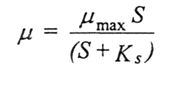
where µmax is the maximum specific growth rate of the organism and Ks, the saturation constant, is a measure of the organism's affinity for the limiting substrate. Ks is the substrate concentration at which the organism grows at half-maximal rate. If cultivation in a chemostat is prolonged, the microbial population adapts to its environment by mutation and natural selection, resulting in the appearance of new, advantageous strains. The competitive advantage of an advantageous mutant relative to the parental strain can be quantified by calculating the selection coefficient (s):
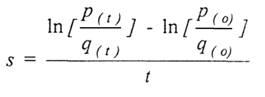
where, p(t) is the concentration of the mutant at time t, q(t)
is the concentration of the parental strain at time t, and p(0) and q(0)
are the initial concentrations of each strain. The selection coefficient is a
measure of the extent to which selection is acting to reduce
the relative contribution of a given phenotype to the next generation. It is a
number between zero and one.
If s = 1, selection against the phenotype is total, and it makes no contribution to the next generation. If s = 0, there is no selection against the phenotype at all (=selectively neutral compared to the favoured phenotype). For example, if the favoured phenotype produces 100 viable progeny, and the alternative phenotype produces only 90, then s = 0.1 and there is adverse selection pressure against the alternative phenotype. An alternative way of expressing this is to describe the fitness of the favoured phenotype as 1.0 and that of the alternative phenotype as 0.9.
Selective advantages for mutants appearing in chemostat cultures have been divided into two main categories:
- mutants which have a higher maximum specific growth rate (µmax) than the parental strain and,
- mutants which have a lower saturation constant (Ks) for the limiting nutrient than the parental strain.
The former (µmax) mutants are usually selected at high dilution rates in a chemostat or turbidostat, and Ks mutants are usually selected at low dilution rates in a chemostat.
In contrast to advantageous mutations, mutations that confer neither a selective advantage nor disadvantage to the mutant relative to the parental strain (neutral mutations) accumulate very slowly in the population (maximally at the forward mutation rate) and never attain high concentrations unless they are linked to an advantageous mutation. Periodic decreases in neutral mutant population have been observed in bacterial cultures maintained in the chemostat for very long periods. This phenomenon is referred to as periodic selection. It is possible to study periodic selection in F. venenatum because the fungus produces macroconidia which are formed from uninucleate phialides; consequently, the nuclei of macroconidia harvested from a culture provide a sample of the nuclei present in the mycelial biomass, and periodic selection can be followed in Fusarium venenatum by monitoring neutral mutations occurring in macroconidia (Fig. 38).
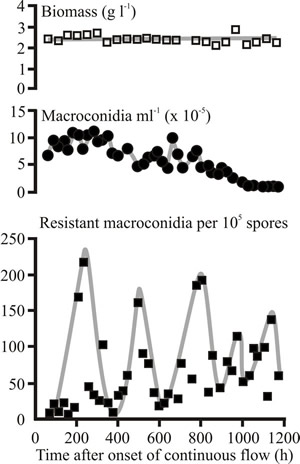 |
Fig. 38. Periodic selection in a glucose-limited laboratory chemostat culture of Fusarium venenatum. Concentrations of biomass, total macroconidia and macroconidia spontaneously resistant to 250 µM cycloheximide in a glucose-limited chemostat culture of F. venenatum A3/5 grown at 25°C and pH 5.8 at a dilution rate of 0.10 h-1 on modified Vogel's medium. The decreases in the concentrations of spontaneous cycloheximide resistant macroconidia in the population are associated with the appearance of other advantageous mutants of unknown phenotype. This phenomenon is known as periodic selection and similar observations have been made with Aspergillus spp. |
|---|
Although neutral mutants accumulate at a linear rate in a chemostat, their concentration decreases when an advantageous mutant arises that does not carry the neutral mutation. This phenomenon of periodic selection provides a means of determining when advantageous mutants appear in a population even when the phenotype of the mutant is not known. For example, the appearance of advantageous mutants in chemostat populations of Fusarium venenatum A3/5 has been determined by monitoring increases and decreases in the levels of chlorate and cycloheximide-resistant macroconidia in the population; at least three advantageous mutants of unknown phenotype appeared in the glucose-limited chemostat population of F. venenatum A3/5 shown in Fig. 38.
In other glucose-limited chemostat cultures of Fusarium venenatum A3/5 that were grown at a dilution rate of 0.19 h-1, periodic selection occurred once every 124 h or 34 generations (Weibe et al., 1993, 1994, 1995). In a glucose-limited chemostat culture of F. venenatum A3/5 grown at a dilution rate of 0.05 h-1 (doubling time of 13.9 h) the Ks values of some of the populations were determined and found to decrease with evolution of the culture. Thus, growing F. venenatum A3/5 in a glucose-limited chemostat at a low dilution rate results in the selection of advantageous mutants of unaltered mycelial morphology but which have a higher affinity for the substrate than the parental strain, which might mean that they have more efficient uptake systems for glucose.
In experiments with glucose-, ammonium- and magnesium-limited chemostat cultures of Fusarium venenatum A3/5 grown at dilution rates of 0.18 or 0.19 h-1 colonial mutants were first detected in glucose-limited cultures at 360, 386 and 421 h (99, 106 and 115 generations) after the onset of continuous flow, and in ammonium- and magnesium-limited chemostat cultures at 447 h (115 generations) and 260 h (71 generations) respectively after the onset of continuous flow. Whenever the evolution of these cultures was allowed to progress, the colonial mutants eventually formed more than 90% of the total population.
Experiment shows that the highly branched phenotype is not responsible for the selective advantage of colonial mutants. Rather, the advantage seems to result from a metabolic alteration which has a pleiotropic effect on branching (Simpson et al., 1998). The development of strategies to prevent or delay the appearance of colonial mutants in industrial mycoprotein™ fermentations is of considerable economic importance. Three possible strategies have been identified so far:
- operating the fermenter at a low dilution rate,
- periodically changing the selection pressure in the fermenter, and
- isolating, or genetically-engineering, sparsely-branched strains of F. venenatum which are more stable than A3/5, at least as far as mycelial morphology is concerned.
However, in the production process, advantages gained by delaying the appearance of colonial mutants by operating the fermenter at a lower dilution rate would be offset by the decreased rate at which biomass is produced under these conditions. Similarly, periodically changing selection pressure introduces unwelcome complication to the production process with a potential for varying the chemical content of the product. However, it is possible to select variants of Fusarium venenatum which are morphologically more stable than A3/5; in particular, in laboratory scale glucose-stat trials with diploid strains of A3/5, colonial mutants did not appear until 1,957 and 2,028 h after the onset of continuous flow, timings which are equivalent to 540 and 594 generations, compared to the 99 to 115 generations for the haploid A3/5.
Premier Foods bought RHM in March 2007, and in January 2011, Exponent Private Equity and Intermediate Capital Group (EPE & ICG) purchased Quorn mycoprotein products and Cauldron tofu products from Premier for £205 million. In 2015, Monde Nissin, one of the leading food consumer goods companies in the Philippines, agreed to purchase all of Quorn Foods from EPE & ICG for £550 million [https://www.mondenissin.com/]. Today, both product ranges are sold in the UK by Quorn Foods under the Marlow Foods Ltd banner. Overall sales of meat-free products in the United Kingdom rose in value from £468 million in 2005 to £549 million in 2009 and were projected to grow to £673 million in 2015. Quorn Foods had a turnover in 2010 of £128.8 million (£16.1 million operating profit) and had around 600 employees across manufacturing sites in Belasis on Teesside (where Quorn is produced by fermentation); Stokesley, North Yorkshire (where Quorn is formed and packed) and Methwold in Norfolk (where cooking and packing of ready-meals takes place). Quorn is now in the Philippines [https://www.mondenissin.com/news/title/quorn-world-leader-in-meat-free-food-is-now-in-the-philippines], and the product website is truly international [https://www.quorn.com/].
However, Quorn’s EU patents, some of which were first filed in 1985, expired in 2010. Although it will be difficult to compete with the present owner’s 30-plus years’ experience with the fermentation method, fermentation tower and related equipment, a potential competitor company claims to have developed a new biotechnology process to produce mycoprotein cheaper than ever before and with zero waste. 3f BIO is a technology spinout from the University of Strathclyde which is producing a mycoprotein product called ABUNDA® [https://www.enough-food.com/]. Its own patented technique involves integrating the production of bioethanol with the fermentation of mycoprotein. The Marlow Foods method for producing mycoprotein uses glucose as a feedstock, whereas 3f BIO’s process uses hydrolysed starch from one or more cereal grains. According to European Patent No. EP3209789B the 3f BIO invention relates to an integrated method for producing and isolating mycoprotein produced by Fusarium venenatum on hydrolysed starch feed stock followed by production of ethanol from the spent partially fermented broth by ‘a micro-organisms(s)’, presumably yeast(s) [sources: www.foodmanufacture.co.uk/ (2011); www.theengineer.co.uk/ (2017)]. 3F BIO Ltd submitted a Generally Recognised as Safe (GRAS) notice (GRN 945) to FDA in 2020 “largely based on the fact that this product as produced by the notifier is substantially the same in composition to mycoprotein that is currently on the market [= Quorn mycoprotein], which is manufactured by the previous notifier (GRN 91), under identical conditions of intended use, using the same strain and through an equivalent manufacturing process.”
Another process uses edible filamentous fungi to convert a cheap and low-nutritional value by-product of the pea-processing industry, into what is called “a vegan-mycoprotein” concentrate for human food applications (Souza et al., 2018).
The expectation of major market success for mycoprotein in the near future is very good news for the whole world. Why this is so derives from the arithmetic that compares the size of the human population (constantly increasing) with the amount of agricultural land available to feed that population (constantly reducing as we build on it, and perhaps reducing further as the climate changes). As it stands, the Earth does not have enough land for all its human inhabitants to enjoy an ‘affluent’ diet; which is one that includes meat from the billions of animals (see Section 17.20) that are farmed each year. This is because so much agricultural land is devoted to feeding those animals with grasses and cereals and conversion of these crops to meat-protein is very inefficient. Assessment of the environmental impacts of food production in general is a growing concern (Poore & Nemecek, 2018; Willett et al., 2019), but meat-free alternatives produced by industrial fermentation, like mycoprotein, would certainly safeguard the Earth’s resources while satisfying our taste for meat-like products.
Updated May, 2021
