13.15 Ectomycorrhizas
Ectomycorrhizas are generally considered to be the most advanced symbiotic association between higher plants and fungi, because although they involve only about 3% of seed plants, all of these are woody species; trees and shrubs, including the majority of forest trees. So the ectomycorrhizal association is globally important because of the large area covered by the plants, and because of their economic value as the source of timber. In total, 43 plant families and 140 plant genera have been identified as forming ectomycorrhizas. In the northern temperate regions, plants such as pine (Pinus), spruce (Picea), fir (Abies), poplar (Populus), willow (Salix), beech (Fagus), birch (Betula) and oak (Quercus) typify the ectomycorrhizal association (or the related ectendomycorrhiza). In the southern hemisphere Eucalyptus and Nothofagus (Southern Beech) are important genera as is the family Dipterocarpaceae, which dominate the lowland rainforests around the globe from South America, through Africa to south-east Asia. This ectomycorrhizal group is reasonably homogeneous, but a subgroup, ectendomycorrhizas, has been separated out (see below). The characteristic features of the ECM association are:
- the presence of a substantial sheath of fungal tissue around the plant root;
- roots which are shorter and wider than uninfected ones, and
- an extensive network of intercellular hyphae penetrating between epidermal and cortical cells, called the Hartig net.
In this association the plant root system is completely surrounded by a sheath of fungal tissue which can be more than 100 µm thick, though it is usually up to 50 μm thick. The hyphae penetrate between the outermost cell layers of the root forming what is called the Hartig net (Fig. 5; Fig. 11). From this a system of hyphal elements (hyphae, strands and rhizomorphs) extend out to explore the soil domain and interface with the fungal tissue of the root.
|
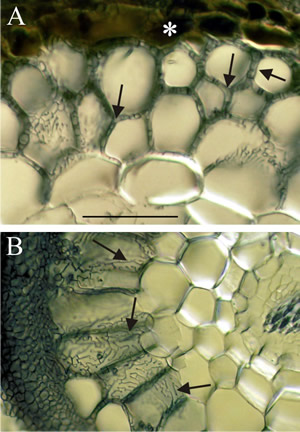
Fig. 11. Light micrographs of hand sections of ectomycorrhizal forest tree roots cleared and stained with Chlorazol black E and viewed with (Nomarski) interference contrast microscopy. Fungal hyphae penetrate between host cells and branch to form a labyrinthine structure called the Hartig net. Host responses may include polyphenols (tannin) production in cells and the deposition of secondary metabolites in walls. Ectomycorrhizal gymnosperms, such as Pinus and Picea, have Hartig net hyphae extending deep into the cortex; this contrasts with the typical situation in angiosperms (such as Populus, Betula, Fagus, Eucalyptus, etc.), which usually have a one cell layer Hartig net confined to the epidermis. A, transverse section of ectomycorrhizal Tsuga canadensis (Hemlock) with labyrinthine Hartig net hyphae (arrows) penetrating between the cortical cells of the root and completely surrounding many cells; note tannin-filled epidermal cells in the inner mantle (asterisk); scale bar = 100 µm). B, root cross section of ectomycorrhizal Populus tremuloides (Quaking or Trembling Aspen) showing labyrinthine Hartig net hyphae (arrows) around elongated epidermal cells. This complex hyphal branching pattern is thought to increase the fungal surface area in contact with the root. The active mycorrhizal zone occurs several mm behind the root tip (because of the time required for mycorrhizal formation), but Hartig net hyphae senesce in older regions further from the root tip. The images are modified from Brundrett, Murase & Kendrick, 1990 and were kindly supplied by Dr Mark Brundrett, School of Plant Biology, University of Western Australia. For more information and illustration of mycorrhizas visit Mark Brundrett’s website at http://mycorrhizas.info.For more information and illustration of mycorrhizas visit Mark Brundrett’s website at http://mycorrhizas.info. |
|---|
A wide range of fungi form ecto- or ectendomycorrhizas: at least 65 genera comprising between 5 000 and 6 000 species of fungi have been identified. About two-thirds of these belong to the Basidiomycota, the rest are Ascomycota except for the genus Endogone, which is a small zygomycetous truffle that forms arbuscular mycorrhizas as well. About three-quarters of the species produce epigeous (above-ground) fruiting bodies, but up a quarter have hypogeous (underground) fruiting bodies such as truffles; members of the Ascomycota are particularly well represented by hypogeous forms. Ectomycorrhizal fungi and include common woodland mushrooms, such as Amanita spp., Boletus spp., Tricholoma spp. Ectomycorrhizas can be highly specific (for example Boletus elegans with larch) but they generally do not show a high degree of host specificity (for example Amanita muscaria with 20 or more tree species as varied as birch, eucalyptus, spruce and Douglas fir). In the other specificity direction, forty fungal species are capable of forming mycorrhizas with pine, and Norway spruce (Picea abies) can form ectomycorrhizal symbioses with over 100 different fungal species. It is common to find mycorrhizas belonging to several different fungi on the root system of a single tree.
Ectomycorrhizas start to develop when hyphae infect the secondary or tertiary roots of woody species, which they seem to prefer, especially on trees. Hyphae grow back up the root from just behind the root cap and meristem, forming a weft that may later become the bulky sheath (Fig. 11). Hyphae from the sheath grow inwards between epidermal cells and cortical cells, by forcing their way mechanically and by excreting pectinases. This forms the Hartig net. Hyphae never penetrate into cells (but see ectendomycorrhizas, below) or into the stele. The intercellular Hartig net may surround each cell completely (Fig. 11), so they have little or no contact with other plant cells. The extensive surface area of the Hartig net is the main interface for exchange of substances between plant and fungus.
Fungal infection changes the growth pattern of the root. The fungal sheath reduces the rate of cell division at the root tip, slowing elongation of the cells and therefore of the root growth. Cortical cells elongate radially resulting in the infected root appearing short and thick compared to uninfected ones (they are often called ‘short roots’; Fig. 12).
The fungal sheath also suppresses root hair development with the result that all nutrients entering the plant must be channelled through the sheath. A fungus will extend its sheath with a growing root, ensuring the root is always covered and preventing colonisation by other fungi. However, fungi can be replaced as roots resume growth after winter. If the fungal sheath does not resume growth straight away, the root can be exposed to other fungal symbionts. This sort of replacement (or supplementation) is different from the replacement mentioned above in relation to early and late stage colonisers.
 |
|---|
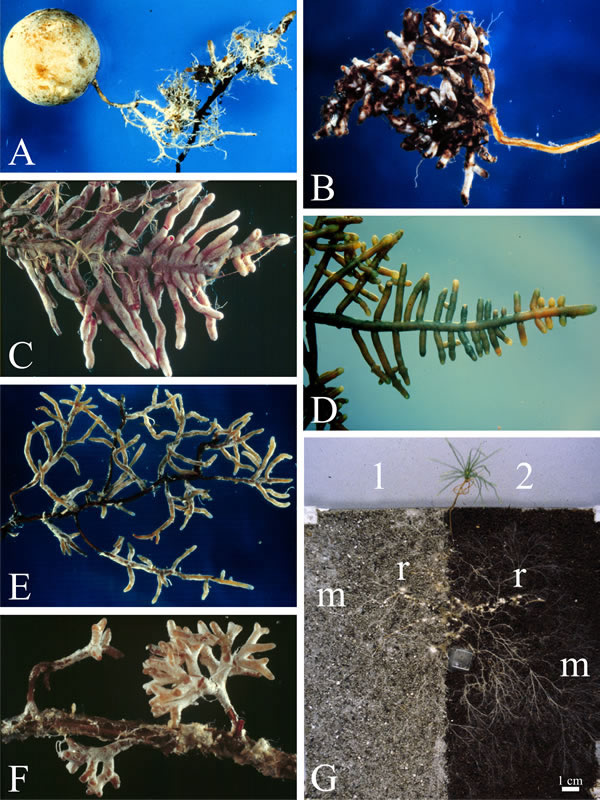
| Fig. 12. Ectomycorrhizal roots; small parts of the root systems various forest tree species to illustrate the morphological diversity of ectomycorrhizal roots. A to D are all roots of Douglas Fir (Pseudotsuga menziesii), but mycorrhizal with different fungi: A, with the basidiomycetous truffle Hysterangium (a truffle fruit body is shown; photograph by B. Zak); B, mycorrhizal fungus = Rhizopogon vinicolor (Boletales, Basidiomycota); C, mycorrhizal fungus = Poria terrestris (= Byssoporia terrestris, Polyporales; B. photograph by Zak); D, mycorrhizal fungus = Lactarius sanguifluus (Russulales; photograph by B. Zak). E and F show mycorrhizas involving the same fungus (Amanita muscaria) but different hosts (photographs by R. Molina): E, Sitka Spruce (Picea sitchensis) and F, Monterey Pine (Pinus radiata). Images A to F prepared from graphics files kindly provided by Dr Randy Molina, Pacific Northwest Research Station, USDA Forest Service, Oregon, USA. Illustration G shows a pine seedling grown in symbiosis with the ectomycorrhizal fungus Suillus bovinus in an experimental microcosm (where the seedling is grown in soil placed in a container made from two sheets of glass separated by a one cm thick former. In this case two soils were used: 1, is from a podzol E horizon soil (explained below), and 2 is a loamy organic soil. The extraradical fungal mycelia (m; evident mainly as hyphal strands) extend from the colonised root tips (r) into both soil substrates and are far more extensive than the roots (scale bar = 1 cm). Podzols are the typical soils of coniferous, or boreal, forests in the northern hemisphere and eucalypt forests and heathlands in the southern. In podzols organic material and soluble minerals are leached from the upper layers (horizons) to the lower; the E horizon is a 4-8 cm thick layer of heavily leached soil and is largely composed of insoluble minerals. The high abundance of mycelium in this mineral soil shows that it is an important growth substrate for ectomycorrhizal fungi. Ectomycorrhizal fungi modify their chemical environment through local acidification around the hyphae and by exuding metal-complexing weathering agents such as organic acids (see Figs 1.2, 1.4, 1.5) and play a central role in mineral weathering of boreal forest soils (Rosling et al., 2009; this image reproduced with permission from Elsevier). For more information and illustration of mycorrhizas visit Mark Brundrett’s website at http://mycorrhizas.info. |
The ectomycorrhizal association is a mutualistic symbiosis; the partners are reciprocally benefited. The biological function of this symbiosis is the exchange of fungus-derived mineral nutrients for plant-derived carbohydrates; photosynthetic carbohydrates are translocated from the plant to the fungus which uses this rich carbon source to develop extensive hyphal growth into the soil. The mycelium explores the soil and absorbs minerals and water which it shares with the plant roots, and also confers pathogen resistance to the plant partner and protection from water-stress. This external mycelium is called the extraradical mycelium. It is an interconnected three-dimensional network of hyphae and, in many cases, specialised hyphal aggregates like strands and rhizomorphs, that not only massively increases the absorbing surface area of the host plant but also the overall potential reach of the host (Fig. 12G), finding and transporting nutrients from distant sites of capture to the sites of nutrient transfer within the mycorrhizal tissues of the host.
The exchange of metabolites is essential for the persistence of both plant and fungus, particularly in stressed environmental conditions. Most ectomycorrhizal fungi are so uncompetitive as saprotrophs that they depend on the plant host for carbon sources. With few exceptions (Tricholoma fumosum being one), the fungi are unable to utilise cellulose and lignin. On the other hand the fungus is able to capture nutrients, particularly phosphate and ammonium ions (and water), which the root cannot access and this results in greatly enhanced mineral uptake for the plant. Success of this symbiosis is essential to the stability of forest ecosystems, trees grow poorly when they lack ectomycorrhizas. Ectomycorrhizal infection is necessary for the successful establishment of some trees, such as Pinus, and can allow seedlings to compete against mature trees in less favourable conditions, such as when the seedling is shaded by the mature canopy leaves.
Ectomycorrhizas can link together groups of trees (the submerged mycelium acting as what has been described as a ‘wood-wide-web’ (Wohlleben, 2017; or Common Mycorrhizal Network, Gilbert & Johnson, 2017) in which signals, as well as nutrients, are exchanged between plants through their mycorrhizas (see also Section 13.17). These networks can transport signals produced by plants in response to herbivore and pathogen infestation to neighbouring plants before they are themselves attacked. There does seem to be a finely tuned balance of signals between the partners at several levels. The speed of transfer to uninfected plants is such that the mechanism could have benefits for plant protection if the networks could be harnessed for pest management in agriculture (Gilbert & Johnson, 2017). Fungal hyphae and plant root cells have developed cell-anchor receptors and mobile signal-ligands like adhesins (Dranginis et al., 2007; see Section 6.8) as mutual sensing molecules, and responses to these signals allow both to adapt rapidly to changes in their local cellular environment and to signal their nucleus through appropriate transduction pathways. As an aside, it’s interesting to point out that this is just one aspect of what is turning out to be a rich sensory environment for plants. Recent research has shown that flowers (of Oenothera) increase nectar sugar concentration in response to the sound vibrations of approaching pollinator insects (Veits et al., 2019), whilst in Arabidopsis, sound vibration treatment triggers various molecular and physiological changes in the plants, some of which boost plant defences against the pathogenic fungus Botrytis cinerea (Ghosh et al., 2019).
On both sides, of course, there is a genetic component to the ability to form a symbiosis, and a panel of responses to environmental conditions which influence (enhance or repress) establishment of the relationship. Cell sensing of the environment and cell-to-cell communications within the ectomycorrhizal tissues must play a major role in this transaction. Some genomics research on symbiotic signalling in an endophytic association (Vasiliki et al, 2019) is described briefly in Section 13.19, and similar research with a lichen (Armaleo et al, 2019) appears in Section 13.18.
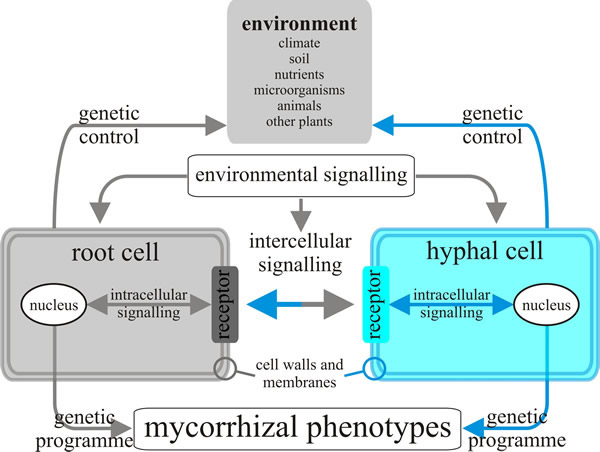
Research on the genetics of mycorrhizal networks has advanced greatly in recent years as molecular tools have increased their resolution and throughput, but a large gap remains between our understanding of the genes and transcripts on the one hand, and the physiology, ecology and evolution of mycorrhizal networks in our changing environment on the other (Tagu et al., 2002; Simard et al., 2012) (Fig. 13).
 |
|---|
| Fig. 13. Model of the inter- and intracellular communications that might exist between fungal hyphae and root cells in the ectomycorrhizal symbiosis. Changes in environmental conditions may produce signals sensed by cells of both partners in the symbiosis, and both probably transduce this information to their nuclei to provoke modifications in gene expression and consequently in phenotypes. Redrawn and modified from Tagu, Lapeyrie & Martin, 2002. |
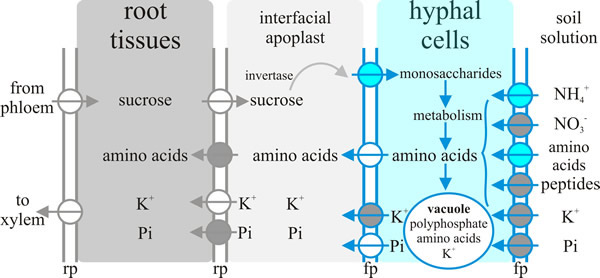
Arguably the most important communication between plant and fungus in the ectomycorrhiza is the exchange of nutrients. As with the exchange of nutrients between endomycorrhizas and their hosts (see discussion and Fig. 8, above) nutrient exchange between fungus and host depends on one partner releasing nutrient into the apoplastic interface and the uptake of that nutrient from the apoplastic interface by the other partner. A model showing the transporters either known or hypothesised to be involved in ectomycorrhizal tissues is summarised in Fig. 14. Note that the system operating at the plant-fungus interface, which is the Hartig net, involves:
- Delivery of sucrose into the apoplast by the plant and its hydrolysis by a plant-derived acid invertase; the resulting hexoses then being taken up by the fungal cells.
- Fungal Pi (inorganic phosphate) uptake systems ‘harvesting’ Pi from the environment, probably assisted by several excreted fungal enzymes (like phosphatases) that could play crucial roles in improving P availability by digesting soil reserves of ‘bound’ phosphate. The hyphae translocate P by active processes (highly mobile vacuoles?) to deliver to the apoplast for take up by their P-deficient host plant.
- Potentially nitrate, ammonium and peptide uptake into the fungus through specific fungal uptake systems feeding intermediary metabolism that supplies amino acids for release into the apoplast by the fungus through amino acid exporters, followed by subsequent amino acid uptake by the plant cell.
- High affinity active K+ uptake by peripheral hyphae from the soil solution and its release through fungal outward K+ channels into the apoplast for absorption by (currently hypothetical) host plant K+ importers.
 |
|---|
| Fig. 14. Nutrient exchange between fungus and host depends on one partner releasing nutrient into the apoplastic interface and the uptake of that nutrient from the interfacial apoplast by the other partner. This diagram summarises current ideas about the transporters acting in ectomycorrhizal tissues that achieve this nutrient exchange. Key: fp = fungal plasma membrane, rp = root plasma membrane. The circles represent transporters, with the arrows indicating direction of transport. Blue circles represent transporters where at least one member of the transporter family has been characterised by functional complementation of a yeast deficient strain; grey circles are putative transporters for which candidate genes exist in the genome; white circles represent hypothetical transporters. Redrawn and modified from Chalot et al. 2002. |
Ectomycorrhizal root systems, meaning plant and fungal partner together, can receive about half of the photosynthetically fixed carbon produced by the aerial parts of the plant. Monosaccharide uptake capacity and carbohydrate flux through glycolysis and intermediate carbohydrate storage pools (trehalose and/or mannitol) in the fungus is greatly increased at the plant-fungus interface to enable such a major carbohydrate flux. Depending on the identity of the fungus involved in the symbiosis, fine control of fungal carbohydrate uptake and metabolism seems to be determined either by developmental mechanisms or by the apoplastic sugar content. Trees increase their photosynthetic capacity to satisfy the increased carbohydrate demand of the symbiosis. In addition, host plants control and restrict carbohydrate flux towards their mycorrhizal partner to avoid fungal parasitism, though the mechanisms responsible for this are still largely unknown (Nehls et al., 2010).
Updated January, 2020