5.18 Cytokinesis and septation
The hyphal growth form of filamentous fungi is an adaptation to the active colonisation of solid substrata. By hyphal extension and regular branching the fungal mycelium can increase in size without disturbing the cell volume/surface area ratio so that metabolite and end-product exchange with the environment can involve translocation over very short distances. Growth of the mycelium is regulated, of course, and three mechanisms involved in regulating the growth pattern of undifferentiated mycelia are regulation of:
- hyphal polarity,
- branch initiation,
- the spatial distribution of hyphae.
Fungal hyphae are variable between species, but generally speaking the hyphal filament, when separated into compartments by cross-walls, has an apical compartment which is perhaps up to ten times the length of the intercalary compartments.
The septa which divide hyphae into cells may be complete (imperforate), penetrated by cytoplasmic strands, or perforated by a large central pore. The pore may be open (and offer little hindrance to the passage of cytoplasmic organelles and nuclei), or may be protected by a complex cap structure derived from the endoplasmic reticulum (the dolipore septum of Basidiomycota). In Ascomycota, the pore may be associated with, perhaps plugged by, Woronin bodies, which are modified peroxisomes. Septal form may be modified by the hyphal cells on either side of the septum, and may vary according to age, position in the mycelium, or position in the tissues of a differentiated structure. These features make it clear that the movement or migration of cytoplasmic components between neighbouring compartments is under very effective control. The hypha is divided up by the septa, and the cellular structure of the hypha extends, at least, to its being separated into compartments whose interactions are carefully regulated and which can exhibit contrasting patterns of differentiation.
Septation in fungi involves chitin deposition in a ring defined by a pre-formed ring of actin microfilaments. Early research showed that septation in the main hypha is in some way defined by the position of the dividing nucleus, though neither septation nor branch formation are universally dependent on orientation of the nuclear division spindle. There is evidence for cytoskeleton-related functions being shared by karyokinesis and cytokinesis and these may form the basis of a structural memory which allows septa to be laid down in positions defined by a nuclear division which occurred some time before. Nuclear migration features in most division processes in fungi, though the coupling between karyokinesis and cytokinesis varies from a rather loose association in hyphae where multinucleate cells are formed to a strict coupling in yeasts and during spore formation when uninucleate spores are produced.
Observations from the 1970s to the present day echo the ancient phylogenetic relationships by emphasising the remarkable similarity between cell cycle events in animals and those in fungi. Primary septa in fungal hyphae are formed by a constriction process in which a belt of microfilaments around the hyphal periphery interacts with microvesicles and other membranous cell organelles. This implies some level of correspondence between fungal septation and animal cell cleavage (cytokinesis). Indeed, genetic analyses of fungal (= yeast), animal and plant cytokinesis have led to the realisation that fundamental mechanisms of cytokinesis may be highly conserved among all eukaryotes (Pollard, 2010). Evidently cytokinesis by septum formation (in hyphae or yeast cells) is quite distinct from the free cell formation we have discussed before (see Chapter 3).
The fundamental function of cytokinesis is to divide both the cell surface and the cytoplasm of one cell to form two cells. The machinery needed for this is assembled towards the end of mitosis at, or close to, the site of nuclear division. Despite the presence of a rigid cell wall, cytokinesis in Aspergillus and both budding yeast and fission yeast occurs by a constriction mechanism resembling that of animal cells. In fungi, as in animals, contraction of an actomyosin-based ring of fibres produces a ‘cleavage furrow’ in the outer membrane that squeezes into the cell (actomyosin is a combination of actin and myosin that, with other substances, makes up the contractile muscle fibres of animals). Microtubules also contribute to the cleavage process in all eukaryotes; in particular, microtubules signal the position of the division plane in animal cells, and elements of this, at least, are conserved in plant cell division even though a constriction process is not evident in plants. This theme of a combination of both similarities and differences extends to the assembly of the contractile ring in yeast and animal cells (Pollard, 2010).
In animal cells, actin and myosin accumulate to the furrow region (and it starts to constrict) during late anaphase. In the fission yeast, Schizosaccharomyces pombe, the actomyosin ring assembles during early mitosis, but cytokinesis does not occur until late anaphase (Gu & Oliferenko, 2015). In budding yeast, Saccharomyces cerevisiae, the actomyosin ring is assembled during two separate stages: myosin first forms a ring at the site of budding at the start of DNA synthesis and then F-actin is incorporated into the ring during late anaphase just prior to contraction.
The cleavage furrow penetrates the whole animal cell to divide it into two daughters. In fungi the contraction is coupled to mechanisms for the synthesis of a cell wall so that the membrane furrow surrounds a septum that grows out from the existing wall, penetrating into the cytoplasm. In yeasts the completed septum separates the bud from its parent (in budding yeast), or two daughters each made up of half the parent cell (fission yeast); whilst in filamentous fungi, septal growth (and cleavage furrow contraction) cease with the septum incomplete, leaving a central pore that may be elaborated in different ways in different fungal groups.
In addition to actin and myosin, cytokinesis requires other proteins, many of which are evolutionarily conserved (Pollard, 2010). Of special importance are proteins called septins, a family of GTPases that form filaments in fungi and animals and are required for assembly of the myosin ring in budding yeast. Septins that localise to the bud neck of S. cerevisiae localise to the cleavage furrows in the fruit fly Drosophila and in mammalian cells, so they are obviously conserved in the two kingdoms. In addition to their role in cell division, septins coordinate nuclear division, membrane trafficking and cytoskeletal organisation (Lindsey & Momany, 2006); although the Coprinopsis cinerea septin Cc.Cdc3 localises to the apices of vegetative hyphae, it is essential for elongation of stipe cells where it assembles into abundant thin filaments that are thought to provide localised rigidity to the plasma membrane of these specialised cells of the fruit body (Shioya et al., 2013). Mutations in budding yeast septin genes result in the loss of several proteins from the bud neck, suggesting that septins might have a structural function there, focussing other components on the cleavage site. Septins can also bind and hydrolyse GTP, so they may also have a role in signalling. Interestingly, IQGAP proteins are another conserved family involved in cytokinesis. IQGAP family members have a structure that includes a domain which binds actin filaments, ‘IQ repeats’ which bind calmodulin, and binding sites for small GTPases, making them candidates as proteins that link Ca2+ signalling to cytokinesis.
Another protein family that is required for eukaryote cytokinesis is the family of formin-homology (FH) proteins. In fission yeast, an FH protein is a component of the actomyosin ring that’s required for recruitment of F-actin to the ring. This protein binds profilin, a protein involved in polymerising actin, so it may act as an initial focus for actin ring formation. A requirement for an FH protein in cytokinesis has been shown in Aspergillus; mutants of the gene (called SepA) lack septa and the protein is required for actin ring assembly in Aspergillus septa. The spindle pole body (SPB) is involved in signalling cytokinesis to the actomyosin ring. A change in SPB biochemistry occurs at the end of spindle elongation, releasing a signal that activates actomyosin ring maturation (F-actin recruitment) and/or contraction, providing a positive signal for cytokinesis initiation.
Microtubule arrays from the mitotic division spindle have functions in cytokinesis (Pollard, 2010). In animal cells, which have a close connection between the position the division spindle and the plane of cytokinesis, signals must pass from the mitotic spindle to the outer membrane of the cell in order to induce furrowing of the membrane to initiate the cleavage furrow. First, at the end of mitosis, the plasma membrane region becomes able to furrow. Then, signals from the mitotic apparatus regulate the contractility of the actomyosin ring system and start assembly of a persistent furrow that eventually bisects the spindle. Specialised subsets of spindle microtubules are important for cleavage furrow positioning, including astral microtubules, which radiate from the spindle poles, and microtubule bundles that assemble in the mid-region of the anaphase spindle and called interzonal microtubules. Two sets of astral microtubules are sufficient to induce furrow formation but there is a continuing requirement for interzonal microtubules for the furrow to deepen (this is called ‘ingression’) and bisect the spindle.
A very similar role for microtubules during cytokinesis in plants is also well established, although plant cytokinesis is achieved by formation of a cell plate that divides the cell into two rather than a cleavage furrow. Nevertheless, vesicle fusion to assemble the cell plate is managed by an array of interzonal microtubules (and actin filaments) called the phragmoplast. So both animal and plant cells reorganise their interzonal microtubules to generate microtubular arrays (called stem bodies in older publications) that perform a specialised function during cytokinesis. It is possible that these microtubules are required for vesicle accumulation and membrane deposition during cytokinesis. Remember that the surface area of the cell needs to increase as it divides, and additional membrane must be recruited into the plasma membrane. At least some of this recruitment derives from insertion of vesicles into the cleavage furrow. Syntaxins are t-SNAREs (see Section 5.11) that are required on the target membrane to promote fusion of the vesicle with the target membrane during exocytosis and some syntaxin-encoding genes have been implicated in cytokinesis. A careful balance in membrane dynamics is required to ensure proper cell surface remodelling (Robinson & Spudich, 2000).
Correct positioning of the cleavage plane is important to ensure that each daughter cell receives a nucleus, so the most economical position is to bisect the axis of chromosome segregation. This fundamental requirement is achieved either by the cleavage plane being specified to a pre-selected site prior to mitosis by a mechanism independent of the spindle, but to which the spindle or cleavage structure is adjusted, or by the division plane being specified by the location of the spindle (as in animal cells). In budding yeast and higher plant cells, the division site is predetermined by a landmark early in the cell cycle (the bud scar in yeast); in fission yeast, the site of cell division correlates with the position of the premitotic nucleus. Sensing cell wall stress is a strategy that enables septation and cell division to be maintained in fungi, even under adverse environmental conditions (Walker et al., 2013).
For the cleavage plane to be specified prior to mitosis and independently of spindle location we have to introduce the idea of some sort of marker being established prior to mitosis that acts as a landmark to dictate the position of both the division and cleavage plane. Budding yeast, Saccharomyces cerevisiae, employs such a mechanism; the division site, which will be the bud neck between mother cell and bud, is selected early in the cell cycle prior to spindle assembly. During mitosis, the spindle is positioned in the bud neck by a cytoplasmic dynein-dependent mechanism involving a dynamic interaction of astral microtubules (microtubules) with the bud cytoplasm and membrane (Figs 9 and 10). The position of the bud site is selected according to a number of ‘landmark’ proteins associated with a bud scar left at the previous bud site.
In Schizosaccharomyces pombe, a medial ring containing actin, myosin II, and other components assembles in the centre of the cell early in mitosis, its location correlating with the position of the premitotic nucleus, which may be located in the cell by the SPB (Chang, 2001). Plants also landmark the location of the division plane prior to mitosis but, here it is the phragmoplast that is directed to the landmark, rather than the metaphase spindle. The site where the new cell plate will fuse with the cell wall is marked by the pre-prophase band (PPB), a temporary array of microtubules whose position is dictated by the location of the pre-division nucleus (Davì & Minc, 2015). During cell division in animal cells, it is likely that spindles are positioned by the concerted action of the forces acting on microtubule arrays; then the cleavage furrow is placed on the basis of the position of the mitotic spindle.
Filamentous fungi form multinucleate hyphae that are eventually partitioned by septa into multicellular hyphae. In Aspergillus nidulans, septum formation follows the completion of mitosis and requires the assembly of a septal band (Fig. 14). This band is a dynamic structure composed of actin, septin and formin. Assembly depends on a conserved protein kinase cascade that, in yeast, regulates mitotic exit and septation (Takeshita et al., 2014).
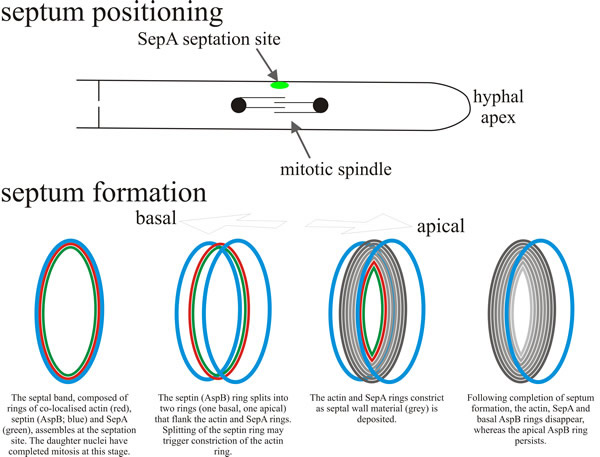 |
| Fig. 14. Schematic model depicting the positioning and assembly of the septal band and the septum. A hyphal segment is drawn at the top, oriented with the tip to the right. In response to signals emanating from the mitotic spindle, SepA localises to the septation site as a cortical dot (green); patches of actin and/or the septin AspB may co-localise with SepA. This locates the site at which the circumferential septal band assembles, composed of co-localised rings of actin (red), SepA (green) and septin (AspB; blue). By this time the daughter nuclei have completed mitosis. Subsequently the septal band assembles the septum. The AspB ring splits into two rings that flank the actin and SepA rings (one on the basal side, one on the apical side). Splitting of the septin (AspB) ring may trigger constriction of the actin ring. As the actin and SepA rings constrict septal wall material (grey in the diagram) is deposited (vectorially synthesised through the plasma membrane (remember, the septum is extracellular; it’s on the outside of the plasma membrane). When assembly of the septum is completed, the actin, SepA and basal AspB rings disappear, leaving the apical AspB ring to persist. Based on a diagram in Harris, 2001. |
However, the crucial feature of filamentous fungi is that their hyphae are multinucleate. Even after the formation of septa, and even in the most regularly-septate fungi, many parts of the hypha are multinucleate. The fact that individual hyphae or hyphal compartments contain several nuclei means that different patterns of nuclear division have been observed in the multinucleated fungal cells. There are three commonly observed patterns: synchronous, parasynchronous and asynchronous, and these vary between organisms and also vary with environmental conditions (Fig. 15). In synchronous division, all nuclei divide simultaneously; in parasynchronous division, mitosis is initiated in one location and then a wave of mitotic divisions travels down the hypha (starting near the apex and proceeding distally) so that nuclei divide in sequence. In asynchronous division, nuclei divide independently of their neighbours, with the result that the spatial and temporal pattern of mitosis in the hypha is randomised.
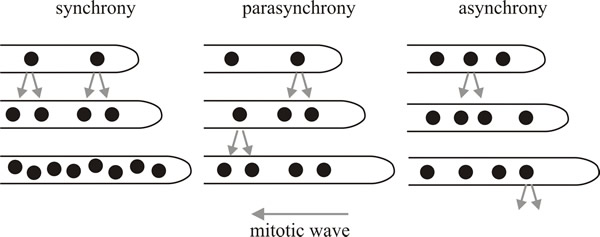 |
| Fig. 15. Patterns of nuclear division in multinucleated fungi. In synchronous division, all nuclei divide simultaneously. In parasynchronous division, mitosis is initiated in one location and then a wave of mitoses travels backwards down the hypha so that nuclei divide sequentially, almost as dominos fall in a line. In asynchronous division, nuclei divide independently of neighbours, so the spatial and temporal pattern of mitosis is randomised. Redrawn from Gladfelter, 2006. |
Nuclei of Neuropora crassa and Ashbya gossypii (both members of the Ascomycota) behave independently of one another so these fungi exhibit an asynchronous division pattern. This type of nuclear division pattern maintains the control of mitosis within a restricted volume of a shared cytoplasm, which potentially means that the hypha is better able to make local responses to nutrients or other environmental stimuli. Asynchronous divisions also allow the hypha to ‘spread the cost’ over a period of time (in terms of the energy and resources required for nuclear replication and division). Additionally, asynchrony might also shield the hypha from the dramatic change in the nucleus-to-cytoplasm ratio that would result if all nuclei divided at the same time (Gladfelter, 2006).
In contrast, synchronous or parasynchronous patterns of nuclear division produce rapid doubling of the number of nuclei in the hypha. Synchronous divisions occur in the apical compartments of vegetative hyphae of the oak wilt parasite, Ceratocystis fagacearum. In Aspergillus nidulans, a parasynchronous wave of nuclear division spreads, in a period of about 20 minutes, from the apex of the leading hyphal compartment. These waves can involve 60 to 100 nuclei, and extend over a length of up to 700 μm of hypha. This wave is unidirectional in A. nidulans, moving away from the hyphal tip, but in Fusarium oxysporum a wave of nuclear division starts at the midpoint of an intercalary (= central) compartment rather than the apical cell, and then the wave progresses in both directions in the cell. Parasynchrony breaks down when it meets a septum. Parasynchrony is also lost when the organism encounters nutritionally poor media, in which circumstances the nuclei divide asynchronously (Gladfelter, 2006).
Coordinated nuclear division cycles of this sort obviously imply an additional, long distance, level of control and regulation. They provide the hypha with a unified response to environmental stimuli, and might also be important for development or morphogenesis. The different nuclear synchronisation patterns observed in multinucleated fungal hyphae leave open the question of how the basic cell cycle control mechanisms are adapted in filamentous fungi. There is evidence that cell cycle control proteins are shared among nuclei in a hypha. Equally, there is evidence that some key cell cycle factors are unequally distributed between nuclei in a hypha. In N. crassa there are some suppressor mutants that suppress mutations in their own nucleus but do not act on neighbouring nuclei sharing the cytoplasm of a heterokaryon.
Also intriguing, are experiments with Basidiomycota in which the two nuclei of a dikaryon are held in an F-actin cage and, apparently, are held a particular distance apart. For example, in Schizophyllum commune, cells in which the two nuclei are 2 μm apart have different patterns of gene expression than cells where the two nuclei are positioned 8 μm apart, though still residing in the same cytoplasm.
Essentially, it comes down to understanding how large cells spatially organise signalling pathways (Gladfelter, 2006). Another aspect is the control and maintenance of cell polarity. Even fission yeast cells have ‘ends’ and a ‘middle’ of the cell, and this sort of axial arrangement depends on the architecture of the cytoskeleton: during interphase in S. pombe microtubules position the nucleus at the middle of the cell and orientate microtubule ‘plus’ ends towards the ends of the cell (Davì & Minc, 2015). This architecture must be reproduced during cytokinesis. Filamentous fungi maintain several (indeed, numerous) axes of polarised growth simultaneously and take the regulation of genes involved the cell cycle and in polarity maintenance to a different level that we still don’t understand (Takeshita et al., 2014; Davì & Minc, 2015; Osés-Ruiz et al., 2016).
Successful cell division requires that cell cycle events are coordinated in time. In particular, cytokinesis must not occur before the mitotic spindle has done its job and the chromosomes have segregated. The synthesis of DNA and the act of cell division are discontinuous processes; a series of ‘events’ that have to occur in a specific sequence and the replication of DNA and segregation of chromosomes to the two daughter nuclei are the two key discontinuous processes for cell survival. To put it simply, if either process is performed inaccurately, the daughter cells will be different from each other, and will almost certainly have lost or gained essential genes or even whole chromosomes. Further, to maintain a constant size during cell proliferation, the growth rate must match the rate of division. Factors that govern proliferation must therefore regulate both the cellular biosynthesis that drives build up of cell mass, and progression through the cell division cycle. Several mechanisms couple cell division to growth and different mechanisms may be used at different times during development to coordinate growth, cell division, and patterning in multicellular organisms so that cells keep a fairly constant size, and developing tissues and organs achieve the proper size and cell density (Neufeld & Edgar, 1998). The potential relationships between cell division and growth identified by Neufeld & Edgar (1998) are:
- cell division drives growth;
- growth drives cell division;
- growth and cell division rates are controlled in parallel by a common regulator;
- independent regulation of cell division and growth.
They conclude that while cell cycle progression appears to be unable to drive
growth, in some cases inhibition of cell division can lead indirectly to growth
suppression (Boye & Nordström, 2003).
The cell cycle, as it occurs in rapidly growing and dividing
eukaryotic cells, is usually described as a series of phases (Fig. 16):
interphase and mitosis (M), with interphase subdivided into the
times when DNA synthesis is going on, known as S-phase (for
synthesis phase) and the gaps that separate S-phase from
mitosis. G1 is the gap after mitosis before DNA synthesis starts, and G2 is the
gap after DNA synthesis is complete but before mitosis and cell division (=
cytokinesis).
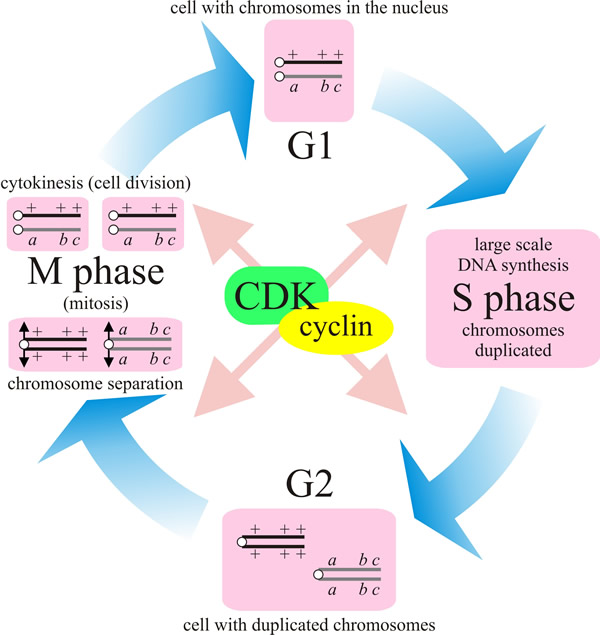 |
| Fig. 16. The different phases of the cell cycle. In the first phase (G1) the cell grows. When it has reached a certain size it enters the phase of DNA-synthesis (S) where the chromosomes are duplicated. During the next phase (G2) the cell prepares itself for division. During mitosis (M) the chromosomes are separated and segregated to the daughter nuclei, and karyokinesis is followed by cytokinesis. This completes the cell cycle and the daughter cells are then back in G1. This diagram depicts a (heterozygous) diploid but applies equally to the mitotic cycle of haploid cells. |
Other descriptors/subdivisions may be used to describe cells that temporarily interrupt their cell cycle; for example, some cells pause after mitosis in a state that is often called G0; they only enter S-phase when they receive signals from outside (for animal cells the signals are usually peptide growth factors). Cells can also pause in G2 phase, in which case the continuation signal causes entry into mitosis (or meiosis in a meiocyte).
The fission yeast, Schizosaccharomyces pombe, normally passes through S-phase immediately after mitosis without a significant lag, and spends most of its cell cycle between the end of S-phase and the next mitosis while the cells grow to the size required for division. The crucial steps in the cell cycle are those that ensure that one process is complete before the next process starts. These are known as checkpoints; safe places to pause the cell cycle for the time needed to check some condition. Different organisms can generate temporary arrest points at different places in the cell cycle while using similar signals to announce continuation. In general, it seems to be possible to construct ‘plug-in modules’ that can detect almost anything that might be relevant to a particular cell at a particular time, and couple this to the cell cycle control machinery. These modules must contain a sensor and an effector, and often lying between these is a signal transduction pathway that depends on protein kinases phosphorylating regulatory proteins. Checkpoints connect together the cell division and chromosome replication cycles so that neither is allowed to run free, and the control circuitry is arranged to produce a strict alternation that prevents cells from returning to mitosis until they have undergone S-phase.
The most revealing contribution to understanding the control of the cell cycle came from genetic studies on yeasts, as we have already mentioned (CLICK HERE to visit the page). While isolating temperature-sensitive mutants for DNA, RNA, or protein synthesis, it became apparent that some mutants were blocked at particular points in the cell cycle. If a population of such a mutant was transferred from 23°C (permissive temperature) to 36°C (nonpermissive or restrictive temperature), the cells stopped growing, but all showed the same morphology; all might fail to produce a bud, for example, or all might produce a bud, but remain attached because of inability to complete cytokinesis. A particularly important one of these temperature-sensitive mutants was called cdc28 (cell division cycle mutant number 28). This fails to form a bud at the nonpermissive temperature, but has no defect in DNA, RNA, or protein synthesis; rather, cdc28 cells continue to grow when transferred to the nonpermissive temperature, and can complete an already started S-phase. The important point is that they can never start a new one. A functional CDC28 gene is required for the cell cycle transition that was called ‘start’ (which is the same as what is called the ‘restriction point’ in higher eukaryotes). Budding yeast halts the cell cycle at ‘start’ in response to several conditions; including nutrient starvation or detection of the peptide pheromone announcing proximity of a potential mating partner.
Many other cdc mutants have been isolated. In the fission yeast, Schizosaccharomyces pombe, a key observation was the recognition of cdc2 as a particularly important regulator of mitosis and a gene essential for cell cycle progression. The first hints of an underlying unity and order to cell cycle control came when Saccharomyces cerevisiae CDC28 and Schizosaccharomyces pombe cdc2 were cloned and sequenced, and found to be homologous and, indeed, interchangeable between these two very distantly related yeasts. The proteins specified by these genes belong to the protein kinase family of enzymes, showing that protein phosphorylation, which is one of the most common ways of altering the activity of enzymes and other proteins generally in eukaryotes, is involved in regulating the cell cycle. Function of the cell-cycle specific protein kinase is activated by a partner protein called a cyclin (because their concentration varies cyclically during the cell cycle). Cyclins are produced or degraded as needed in order to drive the cell through the different stages of the cell cycle. The cyclin forms a complex with its partner cyclin-dependent kinase (CDK)(Fig. 16), which is ‘turned on’ and phosphorylates the appropriate proteins needed for the cell cycle.
The budding yeast cell cycle can be summarised as follows, and the same general principles probably apply to the cell cycles of all eukaryotes (Field et al., 1999):
- at the end of mitosis, mitotic cyclins are degraded by a protease that stays active until ‘start’. This prevents the cell from immediately restarting mitosis.
- At ‘start’, transcription and translation of the G1 cyclins CLN1 and CLN2 is activated, and these proteins combine with Cdc28 to form a protein kinase that initiates formation of the bud and prepares the cell for S-phase (G1 to S transition). This latter includes activation of the genes responsible for cyclins CLB5 and CLB6 that replace CLN1 and CLN2 as partners of Cdc28 to promote DNA synthesis during S phase.
- Towards the end of S phase, synthesis of the ‘late’ cyclins CLB1 and CLB2 starts up, which turns off transcription of the ‘early’ cyclin genes, and as these ‘late’ cyclins accumulate, they transform the Cdc28 kinase into its mitotic form to promote transition from G2 to M.
- The initiation of mitosis in eukaryotic cells is governed by a phosphorylation cascade which culminates in the activation of mitosis promoting factor (MPF).
- MPF consists minimally of the cyclin dependent kinase Cdc28/Cdc2 in yeasts, Cdk1 in higher eukaryotes, and a B-type cyclin regulatory subunit (Ohi & Gould, 1999).
- This takes the cell into telophase, after which the protease is activated, the surviving cyclins are degraded and the cycle finishes.
Updated July, 2019
