5.13 Molecular motors
Microtubules and microfilaments are used by molecular motors for the manipulation and long-distance transport of membranous organelles, vesicles, and RNA and protein complexes (Steinberg, 2000, 2007a; Steinberg et al., 2017). Movement is the responsibility of molecular motors which are molecular machines that move their cargo along F-actin microfilaments or microtubules. Consequently, those motors and their associated transport apparatus deserve a little more detailed attention.
There are three major types of molecular motor: the microtubule-associated kinesins and dyneins, and the actin-associated myosins. As we have mentioned, microtubules are polarised (anchored at the minus end, polymerised at the plus end) and microtubular motors are classified as plus-end-directed or minus-end-directed according to the direction of their movement along the microtubule. Generally speaking (there are exceptions), kinesins and dyneins work in opposite directions, kinesins move their cargo towards the plus end (Fig 6), and dynein walks towards the minus end of the microtubule.
|
|
Fig. 6. Mechanism of kinesin-dependent vesicle transport. Top: A cartoon strip depicting the mechanism of (conventional) kinesin-dependent movement (‘walking’) that transports vesicles along microtubules. Here the transport is from left to right, and is towards the plus end of the microtubule, which is the end with a high polymerisation rate. ATP cleavage leads to repeated cycles of ‘change in shape - detach from the fibre - reattach to the fibre’ alternately in each of the heavy chains making up the motor domain. This results in the coordinated ‘walking’ of the motor along the cytoskeletal fibre, though it’s more of a rapid shuffle than a stylish stroll. You can see animations of this mechanism on YouTube at [https://www.youtube.com/watch?v=y-uuk4Pr2i8] and [https://www.youtube.com/watch?v=-7AQVbrmzFw].
The animation at
https://www.youtube.com/watch?v=wJyUtbn0O5Y is also
highly recommended.
|
Fungal representatives of all of these support the numerous cellular transport processes in hyphae including apical polar extension, septation and nuclear division. The different motors share some structural similarities in that they have a head or motor domain, a linker region, and a tail/stalk region, although there are many differences between them (Fig. 7). In most cases, motors consist of a homodimer of heavy chains and a variable number (up to about 10) of associated lighter chains; the latter often have regulatory roles, and some of them anchor the motor to its cargo (specific details of the components are given below). The heavy chains form the globular motor region, containing the ATPase activity; this region binds either to microtubules or the F-actin of microfilaments, as appropriate. ATP cleavage leads to repeated cycles of ‘change in shape - detach from the fibre - reattach to the fibre’ alternately in each of the heavy chains making up the motor domain. This results in the coordinated ‘walking’ of the motor along the cytoskeletal fibre, though it’s more of a rapid shuffle than a stylish stroll. Nevertheless, the chemical energy provided by ATP hydrolysis is effectively transduced into kinetic energy in the form of the migration of the motors for long distances along their fibre. Yeast dynein can walk along microtubules without detaching, however in metazoans, cytoplasmic dynein must be activated by the binding of dynactin.
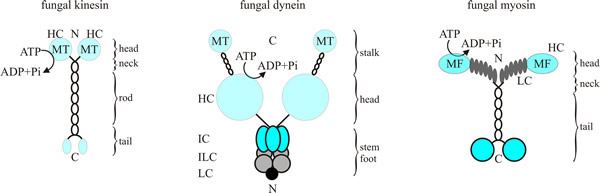 |
| Fig. 7. Diagrams of fungal kinesin (at left), dynein (centre) and myosin (at right). Conventional kinesins from fungi comprise a homodimer of two heavy chains (105 kDa) that provides the enzymatic activity (no evidence for carboxy-terminal light chains that are typical for kinesins from animals has been found). The dynein complex contains two heavy chains of 400 kDa, which probably bind associated polypeptides. In fungal myosins, the heavy chain (180 kDa) of Myo2p, a class V myosin from Saccharomyces cerevisiae provides six binding sites (grey ovals) for a light chain. Abbreviations key: C, carboxyl terminus; HC, heavy chain; IC, intermediate chain; ILC, intermediate light chain; LC, light chain; MF, F-actin-binding site; MT, microtubule-binding site; N, amino terminus. Redrawn and modified from Steinberg, 2000. |
There is a close interrelationship between the actin and microtubule cytoskeletal systems and the same organelles move on both types of filament. For example, mutations in each of these motors can result in similar defects in septation, nuclear migration and organelle distributions in fungi. On the other hand there is also evidence in fungi that movement of some organelles is specific to a particular motor, and which motor does what differs between fungi. For example, in Saccharomyces cerevisiae, transport of mitochondria, secretory vesicles and vacuoles is based mainly on F-actin and its associated myosin motors, whereas in the fission yeast Schizosaccharomyces pombe the microtubular system is involved in this sort of traffic.
Vacuoles are important in transport over long hyphal transport pathways in filamentous hyphae, since they contain many nutrients and essential metabolites, organic and inorganic. In the apical cells of hyphae of most fungi the vacuoles form a highly motile tubular reticulum. The vacuoles are most active in hyphal tips, but non-motile vacuoles at a distance from the tip can be induced to become motile by environmental changes. The vacuolar system in filamentous fungi has been described as ‘…a unique solution to internal solute translocation involving a complex, extended vacuole. In all filamentous fungi examined, this extended vacuole forms an interconnected network, dynamically linked by tubules, which has been hypothesised to act as an internal distribution system…’ (Darrah et al., 2006). There is good evidence that cytoplasmic microtubules are important for the maintenance of vacuolar tubules (Fig. 9), while F-actin microfilaments are not. Staining of hyphae with α-tubulin antibodies shows longitudinal arrays of microtubules. Tubular vacuoles lie parallel to bundles of longitudinal microtubules. Drugs which depolymerise filamentous actin do not affect the vacuole system in hyphae. Tubular vacuoles tend to cluster or accumulate in the apical region except in an apical cap of actin-rich cytoplasm about 5 μm long in the tip hyphae.
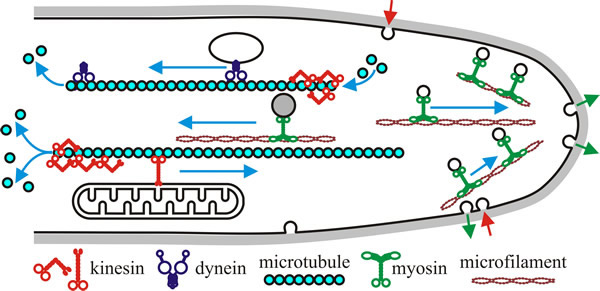 |
| Fig 8. Hypothetical model of motor activity in a fungal hypha. Kinesins and dyneins move in opposite directions along polar microtubules (kinesins towards the plus end that has a high polymerisation rate, and dyneins towards the minus end, the end with low polymerisation rate). Myosins use F-actin (microfilaments), often concentrated in the apex of the hypha. In a classical model, motors translocate their ‘cargo’ (e.g. vesicles and other membranous organelles) along these filamentous tracks. However, some motors influence microtubule stability, probably by modifying the microtubule ends (indicated here by depolymerisation at one end of each microtubule shown). Note that all the indicated processes will be occurring at the same time in a growing hypha, and that there will be a very large number of microtubule and microfilament tracks throughout the hypha. Redrawn and modified from Steinberg, 2000. |
The Saccharomyces cerevisiae genome has genes encoding five myosins, six kinesins and one dynein. This is a modest number compared with some vertebrate systems that have 50 different motors cell. This must mean that each motor participates in several cellular processes in fungi. Fungal motors are known to be involved in the following (and see Fig. 8):
- secretion and endocytosis;
- cytokinesis;
- organelle positioning and inheritance;
- mitosis;
- genetic recombination;
- RNA transport.
Myosins and kinesins belong to large and diverse families of proteins, and a complex nomenclature has developed as they have been identified. Myosin class II is the predominant class of myosin found in muscles and is historically referred to as ‘conventional myosin’; there are another 19 classes of myosin which are currently referred to as the ‘unconventional’ myosins.
Numerous kinesins (more than 600 from a variety of species) have been described; they share a common motor domain of 340-350 amino acids. A prominent member of the kinesin family originally identified in neuronal cells is kinesin-1; this is the ‘conventional kinesin’, which is a plus end-directed motor. Kinesin-1 is not present in Saccharomyces cerevisiae but is involved in hyphal extension of filamentous fungi. Conventional kinesins are two-headed molecular motors that move over micrometer-long distances on their microtubules. Movement over such long distances is called ‘processive movement’ and in vitro assays can be used to measure the processivity of purified motors.
For example, Neurospora kinesin (NKin) moved 1.75 µm on average (n = 182) before detaching from the microtubule, while human kinesin motors moved only half the distance (0.83 µm, n = 229) under identical conditions. A yeast kinesin, Kar3p, translocates at rates of about 1 to 2 µm min-1. In contrast, two kinesins of Saccharomyces cerevisiae that belong to the category kinesin-14 are ‘unconventional’ in that they do not motor to the microtubule plus end like kinesin-1, rather they transport cargo to the minus end of the microtubule. Yet, despite being ‘unconventional’, one is essential for meiosis and mating in yeast, and the other has an important role at the spindle pole body during yeast mitosis. Kinesin-7 and kinesin-1 motors participate in the transport of regulatory compounds to microtubule plus ends and in so doing affect microtubule dynamics and organisation. Kinesin-14 and kinesin-8 motors probably affect the stability of microtubules in mitosis and interphase in fungi; and the minus-end-directed kinesin-14 (and dynein, too) can transport assembled microtubules to particular regions of the cell, so polarising the microtubule array (Steinberg, 2007; Steinberg et al., 2017).
Myosins and, particularly, kinesins both seem to be numerous and functionally specialised, but in contrast there is only one major form of dynein to serve many different cellular roles. Functional diversity for dynein is achieved through the use of an activator or cofactor, dynactin, in most if not all of its functions.
In the next few paragraphs we will briefly mention a few specific examples of motor usage for a range of cellular functions in yeasts and filamentous fungi. Better understanding of mechanisms involved in vesicle migration in fungi might reveal sites for selective toxicity allowing development of new antifungal agents. The overall summary is brought together in Fig. 9.
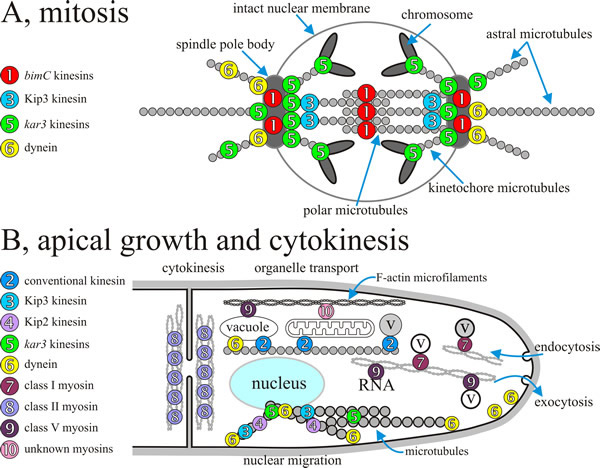 |
| Fig. 9. Overall summary diagrams, combining data from several fungi, indicating the localisation and/or assumed sites of action of specific fungal motors during mitosis and during polarised apical growth of filamentous hyphae. A, during mitosis kar3-kinesins influence the dynamics of spindle microtubules and counteract bimC-like motors, which appear to crosslink polar microtubules. In addition, Kar3 protein might function within the chromosomal kinetochore. Anaphase is supported by cytoplasmic dynein that exerts pulling forces on astral microtubules (see Figs 10 and 11) and, in conjunction with Kip2 protein and Kip3 protein, probably modifies microtubule dynamics. Note that the localisation of Kip3 protein within the spindle is not known. B, molecular motors are involved in a wide range of processes during apical extension and cytokinesis of the hypha; various sorts of organelle transport and positioning are highlighted here (and see Fig. 8), including nucleus, vacuole and mitochondrion, and there is a rapid traffic of a very diverse population of vesicles and microvesicles (all labelled ‘v’ here; compare Fig. 5). Redrawn and modified from Steinberg, 2000. |
One general point that we need to make early on is that although we have given the impression so far that motors move along their microtubules to transport cargo, this is not the whole story. They certainly do move, and this is the processivity that can be demonstrated so readily with in vitro assays. Motors also modify the dynamics of the cytoskeleton; meaning that they affect the lengthening and shortening of microtubules and this delivers cargo, too. The motor domain of some motors might only be needed to reach the final site of delivery at the end of the microtubules. A particular example is the yeast kinesin Kar3p (the symbol ‘Kar3p’ means ‘the protein corresponding to gene sequence Kar3’) that functions in mitosis mainly by destabilising the microtubule at the SPB; that is, Kar3p pulls the microtubule into the SPB. Kar3p also occurs in the kinetochore of chromosomes, raising the possibility that in this case chromosome movement on the mitotic spindle results from the microtubule being pulled in and depolymerised at both ends.
Two other kinesin motors, Kip2p and Kip3p, are also involved in modifying microtubule stability during nuclear migration and mitosis in yeast and similar reports of destabilising activities of spindle motors in vertebrates suggests that modification of cytoskeletal dynamics might be a crucial feature of their cellular function. Similarly, mutants of Aspergillus nidulans, indicate that a dynein motor destabilises and thereby exerts pulling forces on SPB astral microtubules during nuclear migration (Karki & Holzbaur, 1999)(Figs 10 and 11, below). Generally speaking, as well as providing motility, kinesins and dynein also actively participate in organising their tracks in fungal cells. However, the molecular mechanisms by which motors regulate microtubule stability and turnover remain unknown (Urnavicius et al., 2015; Xiang et al., 2015; Steinberg et al., 2017).
After mitosis, cell separation during cytokinesis requires conventional (class II) myosins in S. cerevisiae and S. pombe. In S. pombe, myosin assembles into a ring-like structure at the cell cleavage plane, where it interacts with F-actin and supports cytokinesis.
The cell wall determines the shape of the yeast cell and the hypha in filamentous fungi and its assembly shows extreme polarity (Takeshita et al., 2014). Intracellular transport of vesicles enables the hyphal cell to construct and modify the wall and it’s been calculated that transport to the hyphal apex of up to 38,000 vesicles per minute is necessary for each fast-growing hypha of Neurospora crassa. This is the scale of the process, which is supported by apically-polarised transport of vesicles along both F-actin microfilaments and the microtubular cytoskeleton. Some so-called class V, or unconventional, myosins as well as conventional kinesins take part in this.
Additionally, class I myosins appear to support polarised growth and endocytosis (e.g. Myo3p and Myo5p from Saccharomyces cerevisiae and MYOA from Aspergillus nidulans). However, MYOA is also required for secretion, so this myosin is involved in both endo- and exocytosis in A. nidulans. Exocytosis in S. cerevisiae involves a class V myosin, called Myo2p, which also contributes to polarised growth of the bud. Myo2p binds secretory vesicles with its carboxy-terminal tail and moves its cargo along F-actin filaments to sites of growth. Myo2p has been identified with several functions in organelle trafficking and spindle orientation. At present, Myo2p appears to be the main membrane bound motor in yeast and, is therefore likely to participate in the transport of many components, including the Chs3p chitin synthase protein required for cell wall synthesis. A significant fraction of the total Myo2p in the cell is associated with a large, mRNA-containing particle that is distinct from the exosome and actively translating polysomes. Myo2p may promote the release of mRNAs from this particle for either degradation or translation (Chang et al., 2008).
In some fungi the chitin synthase activity is fused to their molecular motor. Examples include CsmA of A. nidulans and Csm1 from the rice-blast fungus Pyricularia oryzae. These are myosin motor/chitin synthase fusion proteins and both domains are required for correct cellular function. Another class V myosin, Myo4p, is responsible for specifically localising of ASH1 mRNA in the daughter bud of a budding S. cerevisiae cell (Ash1p is a repressor of the mating-switch endonuclease that allows mother cells to switch their mating type; see Chapter 8). Showing that cytoskeletal motors transport mRNA, and by so doing generate RNA gradients in the fungal cell.
Microtubule motors of the conventional kinesin class I have been found in fungi belonging to the zygomycetes, Ascomycota and Basidiomycota. They participate in apical exocytosis generally as well as having other functions (e.g. mitochondrial positioning in Nectria haematococca, and vacuole organisation in Ustilago maydis). The mitotic spindle consists mainly of microtubules and we have already indicated how kinesins can be involved in microtubule dynamics and chromosome movement during fungal mitosis.
Assembly and organisation of the spindle requires counteracting motors to create tension within the spindle rather than moving a cargo; Kar3 and bimC motors have these counteracting functions. Kar3 kinesin motors (specifically, Kar3p of S. cerevisiae; KlpA of A. nidulans; and Pkl1 of S. pombe) are unconventional in that they move towards the minus ends of microtubules; they also locate at the spindle poles where their major role is to destabilise (and ‘reel-in’) microtubules, as mentioned above. Conventional kinesins of the bimC family are required, in all organisms so far examined, for separation of spindle pole body/centrosome (depending on organism) at the onset of mitosis, as well as for the assembly and maintenance of the spindle structure. They may also alter microtubule dynamics, but bimC-like motors have two motor domains at each end and they are located in the middle region of the spindle during anaphase suggesting that bimC kinesins separate the spindle poles by crosslinking the polar microtubules they are destabilising.
Fungal dynein is located outside the mitotic nucleus in the growing apex and serves two general functions; the motor pulls on astral microtubules to drive nuclear migration and it effects transport of exocytotic vesicles. This is an extreme contrast with vertebrate animals in which cytoplasmic dynein is located at the spindle and functions in spindle assembly and chromosome segregation.
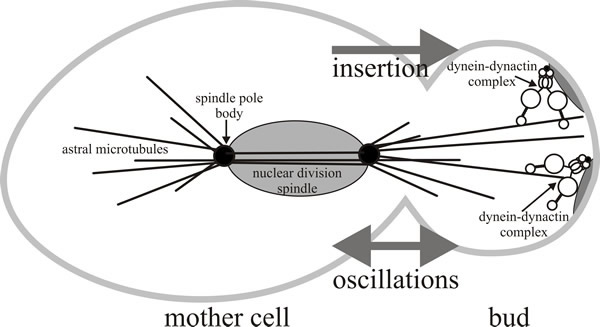 |
| Fig. 10. A model for the role of dynein-dynactin during anaphase nuclear migration in Saccharomyces cerevisiae. The dynein-dynactin complex has been shown to be involved in mitosis in a function that partially overlaps with the kinesin-related protein Kip3p which orients the nucleus near the bud neck (not shown here). Dynein is involved in inserting the nucleus through the bud neck during anaphase, and is also responsible for the oscillatory movements of the nucleus observed during nuclear insertion. Redrawn and modified from Karki & Holzbaur, 1999. |
Cytoplasmic dynein is a multisubunit protein (overall molecular mass about 1.2 MDa) consisting of two heavy chains of about 500 kDa each, which fold to form the two heads of the motor, as well as multiple intermediate chains (about 70-74 kDa each), light intermediate chains (about 53-59 kDa), and light chains (from 8-22 kDa). Dynactin is also a large multisubunit complex of at least seven polypeptides ranging in size from 22 to 150 kDa. Studies with Saccharomyces cerevisiae, Neurospora crassa, and Aspergillus nidulans demonstrate interaction between dynein and dynactin. Specifically, dynactin acts as an anchor that stabilises the dynein so it can pull on astral microtubules coming out of the spindle pole body (Figs 10 and 11) (Xiang, 2018).
In budding yeast, the SPB is embedded in the nuclear envelope, where it serves as the origin for spindle microtubules from its inner ‘spindle plaque’ and cytoplasmic (called astral) microtubules from its outer plaque. Astral microtubules and motors, together with the actin cytoskeleton and additional cell-polarity determinants act to control nuclear movements into and through the neck of buds (Fig. 10). Two major nuclear movements occur during yeast budding: alignment of the nucleus along the mother-to-bud axis and near the neck of the bud, and then the post-anaphase propulsion of the daughter nucleus through the neck and into the bud. The model shown in Fig. 10 suggests that the Kip3 kinesin positions the dividing nucleus near the bud neck, while dynein is responsible for inserting the nucleus through the bud neck during anaphase, and also causes the oscillatory movements of the nucleus observed during nuclear insertion.
Fig. 11 shows a model for the role of dynein and dynactin in nuclear migration in Aspergillus nidulans. Dynein is known to localise to the apex of the hypha, in association with the cell membrane. Another notable feature of filamentous fungi is the even distribution of nuclei along the fungal hypha. Achieving this also requires dynein and dynactin. Cytoplasmic dynein, which is anchored to the cell cortex through dynactin, is located at sites where the nuclei need to be positioned and this ensures the correct positioning of daughter nuclei (Roberts & Gladfelter, 2016).
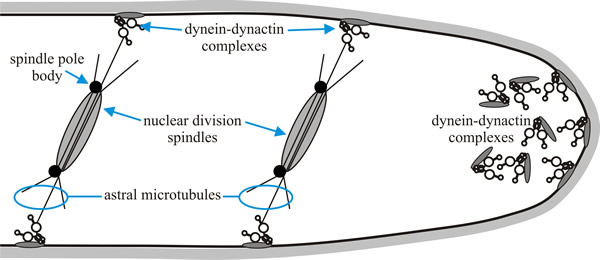 |
| Fig. 11. A model for the role of dynein and dynactin in nuclear migration in Aspergillus nidulans. Cytoplasmic dynein, which is anchored to the cell membrane through dynactin, is located at sites where the nuclei need to be positioned. Dynein is also known to localise to the apex of the hypha. Redrawn and modified from Karki & Holzbaur, 1999. |
Other proteins are involved in specifying the position of the dynactin anchors. One seems to be Num1, a protein defective in one of the first mutants identified in the nuclear migration pathway of budding yeast, which is named for Nuclear migration. Num1 is the cortical anchor for the motor protein dynein. The NUM1 gene encodes a complex, 313 kDa protein which has pleckstrin homology (PH) domains. PH domains were originally identified as an internal repeat in pleckstrin, a phosphoprotein from blood platelets. PH domains are found in animals and fungi, but have not yet been detected in plants or bacteria. Proteins carrying PH domains are either involved in signal transduction or they are part of the cytoskeleton. The ligands for many PH domains are membrane-bound inositol phospholipids, which supports the role of PH domains as membrane anchors (Bloom, 2001). Molecular motors contribute to numerous processes that are of key importance to the organisation and polarisation of extension in fungal cells in yeasts and filamentous hyphae alike (Martin & Arkowitz, 2014; Roberts & Gladfelter, 2016). We will return to this point below [in Section 5.16, CLICK HERE to see it now].
Updated July, 2019