6. In vitro tissue transplantation
Commitment to pathways of differentiation in the Coprinopsis hymenium has been studied by Chiu & Moore (1988a) who removed gill lamellae from caps at various stages of development and placed them onto the surface of nutrient agar explantation medium or 1% water agar. Their further development was then observed during incubation at 27oC. Cytological examination of 16 specimens of explanted lamellae taken at the dikaryotic stage (prior to meiosis) showed that only very few probasidia in some samples proceeded to prophase I, even after 2 days incubation on explantation medium. The majority of probasidia in such samples remained at the stage which they had reached at the time of explantation despite the fact that surrounding cells (at this stage of development, largely tramal hyphae) formed hyphal outgrowths. Probasidia of samples taken at or after prophase I all completed meiosis and sporulation after explantation (25 specimens were tested). In contrast, paraphyses, cystidia and tramal hyphae in the same samples reverted to hyphal growth by formation of one to many hyphal apices.
Thus, although young probasidia are unable to continue development on explantation; they are somehow inhibited from reversion to the vegetative state; that is, they are specified as meiocytes but not yet determined for sporulation. Paraphyses, although highly differentiated by being much swollen, retain the ability to revert immediately to (dikaryotic) vegetative growth on explantation. All the evidence suggests that prophase I is the critical stage at which basidia become determined for the division programme. In the experiments of Chiu & Moore (1988a), similar results were obtained whether water-agar or nutrient-agar was used as the explantation medium. Thus, meiosis in basidia, once initiated, is endogenously regulated and proceeds autonomously.
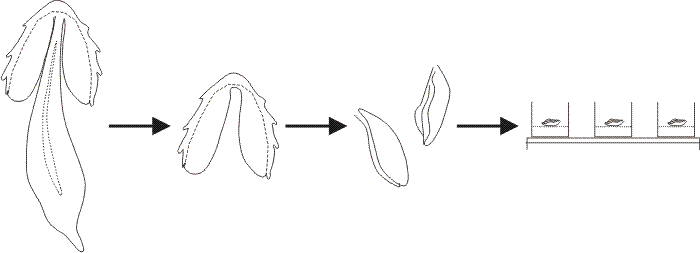 |
| Fig. 28. In a typical explantation experiment gill lamellae were excised from a Coprinopsis cinerea fruit body after the cells of the veil had been scraped off the cap surface. A segment consisting of two or three gills was placed in each experimental well of a multiwell tissue-culture plate. The Meathops dikaryotic strain of C. cinerea was used for the experiments described here; this is a wild type strain collected in Cumbria. The developmental stage of the tissue at explantation was determined by microscopic examination of squash preparations stained with acridine orange. For general purpose assays the gills were excised just at the conclusion of meiosis (i.e. the beginning of sporulation) so that, in control explants to plain buffer agar, spore pigmentation and gill autolysis occurred within 24 h after explantation. This permitted a wide range of compounds to be screened rapidly by simply scanning the wells by eye to detect those in which the gills blackened and autolysed (i.e. normal development, indicating the tested compound to be non-inhibitory), and those in which the gills remained white (i.e. development arrested, indicating the tested compound to be an inhibitor of hymenium development). Modified from Chiu & Moore (1988a). |
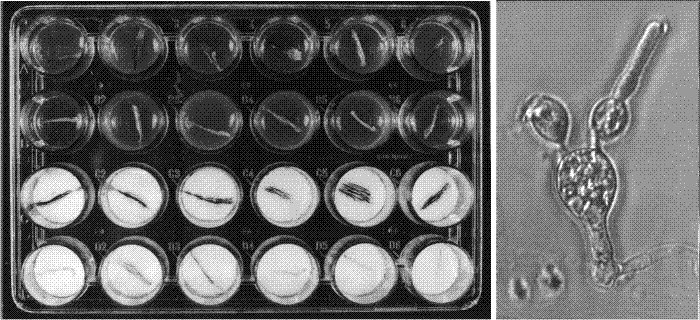 |
| Fig. 29. Sample results of explantation experiments. The explantation technique outlined in Fig. 28 was developed into a routine bioassay, used to study the effects of exogenous compounds on the progress of differentiation of basidia. For the standardised assay we used Nunclon Delta SI 24-well ‘Multidishes’ which have 25 mm internal diameter wells. For tests on agar medium, 0.35 ml of autoclaved agar (buffered to pH 7 except in experiments testing the effect of pH) was placed into each well, and covered with a sterile disk of cellophane after the agar had set. For tests with liquid medium, 0.35 ml of filter-sterilised medium was placed in each well with two sterile 24 mm diameter disks of Whatman GF/D glass microfibre filters and subsequently overlaid with a disk of cellophane. The sample dish, shown after 24 hours incubation at 27° C, under a photoperiod of 16 h light-8 h dark (conditions used to induce fruit bodies). The top two rows of wells contain agar media and the bottom two liquid media, and in each case the lower of each pair of rows contains medium supplemented with 50 mM ammonium chloride. Note that the gills remain white in the ammonium-tests but become covered with pigmented spores in the control media. The micrograph at right shows what happens to an ammonium-inhibited basidium: spore maturation is halted, but a new hyphal apex can form and grow out as a vegetative hypha. Modified from Chiu & Moore (1988b). |
Although basidia appeared to be specified for meiosis and sporulation, these processes were slowed in the explants. Some gills isolated at prophase I had formed only sterigmata after one day, producing spores after two days although karyogamy through to spore maturation normally occupies only 10 h. Thus, explantation and isolation from the environment of the fruit body cap slows the rate of maturation quite considerably. Similarly, an important implication of the ready ability of paraphyses (and tramal hyphae) to revert to vigorous hyphal growth on explantation is that this growth mode must be actively and continually inhibited in vivo to ensure the orderly formation and development of the fruit body. Thus, it is extremely important that a differentiating 'environment' is maintained within the intact tissue, possibly via morphogens, to ensure fulfilment of development. Primordia are often enveloped in an extracellular matrix of mucilage which could serve as the medium through which morphogens could maintain the differentiated state and avoid the dedifferentiation which so readily occurs upon transplantation.
Breaking commitments. The explantation technique of Chiu & Moore (1988a) has been developed into a rapid small-scale bioassay which can be used to study the effects of exogenous compounds on the progress of differentiation of basidia after removal from their parent fruit body (Figs 28 and 29). The technique was adapted to use multiwell tissue culture plates; each well containing one fragment of experimental tissue on a glass-fibre filter disc soaked with 0.35 ml of experimental medium. This adaptation allowed the explantation method to be used to assay the effect of a large number of chemicals on basidium differentiation in vitro. Of a range of compounds tested, only ammonium and glutamine, and some structural analogues, were able to inhibit basidium differentiation 24 h after explantation of the segments. Growth was not inhibited; instead the differentiation inhibitors caused vegetative hyphal tips to grow out from regions of the basidial apparatus expected to be in active growth during sporulation (Fig. 29). Depending on the stage reached at the time of exposure to the inhibitors, vegetative hyphal tips emerged from the four apical sites for sterigmata, from the tips of sterigmata, from partially formed or abnormal spores, and from the basal regions of the basidium from which paraphyses would be expected to arise.
The experiments described above showed that ammonium ions and glutamine could halt meiocyte differentiation in vitro. Chiu & Moore (1988b) showed that ammonium ions and glutamine also halt meiocyte differentiation in vivo. Ammonium salts injected into the caps of young fruit bodies with a microsyringe also terminated further development. Very young primordia (prekaryogamy) were not able to withstand the damage caused by injection and in most cases aborted. From the meiotic division stage onwards, very small volumes could be injected without causing non-specific damage. Injections of 2.5 μl of 1 M ammonium salt solutions (buffered to pH 7) were effective in locally suppressing sporulation if injected in post-meiotic and early sporulation stages, causing the occurrence of white zones around the point of injection as the rest of the cap matured and produced its crop of blackened spores. Similar injections of water or buffer had no visible effect on fruit body maturation.
Some ammonium-treated basidia were apparently merely arrested, but some reverted to hyphal growth. Remember that this behaviour is quite unusual for basidia which characteristically continue sporulation if explanted to buffer agar after karyogamy, and remain arrested but without reverting if explanted before karyogamy (Chiu & Moore, 1988a; Chiu & Moore, 1990c). Thus a further direct effect of ammonium treatment is the rapid and regular promotion of reversion to hyphal tip growth among the basidial cells. This constitutes a breakdown of the commitment normally shown by these cells to their developmental pathway. The pattern of reversion was also interesting as the new hyphal apices were not distributed randomly, rather they formed at sites expected to be involved in active wall synthesis during the normal progress of development. Exactly as described above for the in vitro experiments, when the tissue exposed to ammonium treatment was in post-meiotic and early sporulation stages the reversion hyphae grew out at the sites of sterigma formation; if the basidia had formed sterigmata, hyphae, instead of basidiospores, grew from their apices; if spores were in process of formation, exposure to ammonium caused termination of spore formation and outgrowth of hyphal tips (Chiu & Moore, 1990c). Hyphae also emerged from basal regions of the basidium.
Ammonium inhibited the meiocyte development pathway in vitro when applied at any time during meiosis (stages prophase I through to the second meiotic division were tested). When applied at similar stages in vivo, ammonium retarded the rate of progress through meiosis but did not suppress sporulation. When applied at later sporulation stages (sterigma formation, spore formation, spore pigmentation), ammonium arrested sporulation completely both in vivo and in vitro. Thus, exposure to ammonium causes termination of the normal developmental sequence of the basidium; the meiotic process shows some sensitivity to ammonium-arrest but by far the most obvious ammonium-sensitive stages are the post-meiotic sporulation processes of sterigma and spore formation.
Since ammonium ions cause basidia, the only committed cells of the hymenium, to abort sporulation and revert to hyphal growth, normal sporulation may require some form of protection from the inhibitory effects of metabolic sources of these metabolites. Significantly, the ammonium assimilating enzyme NADP-GDH is derepressed specifically in basidia (Stewart & Moore, 1974), being localised in microvesicles associated with the cell periphery (Elhiti, Moore & Butler, 1987) where it serves as a detoxifying ammonium scavenger. [TOP]
Updated December 7, 2016










