18.3 Clinical control of systemic mycoses for the 21st century: integrated pest management and combinatorial therapy
The antifungal agents that are currently most widely used clinically are the azoles and polyene antibiotics (Table 7), both of which target the ergosterol of the fungal plasma membrane. The principal polyene antibiotics, amphotericin B and nystatin, bind selectively to ergosterol and disrupt the fungal membrane making it leaky. The azole antifungal agents, such as fluconazole and ketoconazole, affect the biosynthesis of ergosterol and this affects membrane composition and function. Other potential targets are noted below.
Table 7. Antifungal agents in
common use at the start of the 21st century |
||||
|---|---|---|---|---|
| Primary target | Detail including nature and names of drugs | |||
| Membrane | Ergosterol inhibitors | Azoles (lanosterol 14-alpha-demethylase inhibitors) | Imidazoles | topical: Bifonazole, Clomidazole, Clotrimazole, Croconazole, Econazole, Fenticonazole, Ketoconazole, Isoconazole, Miconazole, Neticonazole, Oxiconazole, Sertaconazole, Sulconazole, Tioconazole |
| Triazoles | topical: Fluconazole, Fosfluconazole | |||
| systemic: Itraconazole, Posaconazole, Voriconazole | ||||
| Benzimidazoles | topical: Thiabendazole | |||
| Polyene antimycotics (ergosterol binding) | topical: Natamycin, Nystatin | |||
| systemic: Amphotericin B | ||||
| Allylamines (squalene monooxygenase inhibitors) | topical: Amorolfine, Butenafine, Naftifine, Terbinafine | |||
| systemic: Terbinafine | ||||
Wall |
β-glucan synthase inhibitors |
echinocandins (Anidulafungin, Caspofungin, Micafungin) | ||
| Intracellular | Pyrimidine analogues/Thymidylate synthase inhibitors |
Flucytosine |
||
Mitotic inhibitors |
Griseofulvin |
|||
| Others | Bromochlorosalicylanilide, Methylrosaniline, Tribromometacresol, Undecylenic acid, Polynoxylin, Chlorophetanol, Chlorphenesin, Ticlatone, Sulbentine, Ethyl hydroxybenzoate, Haloprogin, Salicylic acid, Selenium sulphide, Ciclopirox, Amorolfine, Dimazole, Tolnaftate, Tolciclate, Flucytosine | |||
| Tea tree oil, citronella oil, lemon grass, orange oil, patchouli, lemon myrtle, Whitfield’s ointment (a mixture of benzoic and salicylic acid used to treat athlete’s foot) | ||||
| Pneumocystis pneumonia treatments: Pentamidine, Dapsone, Atovaquone | ||||
| Other potential targets include: mannoproteins involved in adhesion, translation elongation factor, and proteinases. | ||||
Other potential targets that are evident at present are:
- microtubules; carbendazim (benzimidazol-2-yl-carbamate, Fig. 10) binds to the β-tubulin subunit of microtubules and blocks mitosis. It is used to treat plant diseases caused by ascomycetes, but it is a potential carcinogen. The commercial fungicide is known as benomyl (Fig. 10) and is is hydrolysed within the plant to for the active agent, carbendazim. Griseofulvin (Fig. 10) binds to microtubule associated proteins, affect the microtubule assembly process and thereby disrupt fungal cell division, blocking mitosis at metaphase. It is used to treat topical skin infections including athlete’s foot.
- Dimorphism; the morphological transition between hyphal and yeast-like forms is critical for the pathogenesis and virulence of many fungal pathogens of animals and plants alike. Better understanding of the controls that enable this switch in morphology could reveal potential targets for management of the diseases (Gauthier, 2015; Nigg & Bernier, 2016).
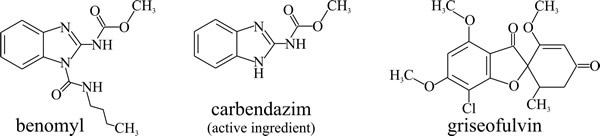 |
|---|
| Fig. 10. Structural formulae of antifungal agents that target microtubule function. |
- Nucleic acid synthesis is also an established target; 5-fluorocytosine (5-FC) was discovered during searches for antileukaemic drugs, it is used to treat systemic fungal infections of man and is effective against Candida but not Histoplasma. Metalaxyl (Fig. 11) an acylalanine fungicide: inhibits RNA polymerase I (responsible for ribosomal RNA synthesis) and is used to treat infections in plants caused by Oomycota (Kingdom Chromista) such as the cause of potato late blight, Phytophthora infestans.
- Sordarins selectively inhibit fungal protein synthesis by interacting with translation elongation factor 2 and the large ribosomal subunit. Sordarins have potent fungicidal activity in vitro against Candida albicans and other Candida species, as well as other yeast-like fungi and filamentous moulds. Unfortunately they have little or no activity against opportunistic filamentous fungi in vivo, though they do have promise for treatment of candidiasis, histoplasmosis, pneumocystosis, and coccidioidomycosis.
A new approach to treatment of invasive aspergillosis, yeasts and other moulds is provided by the first of the so-called second-generation triazoles, voriconazole (Fig. 6 in Section 18.1), which can be used in both intravenous and oral formulations [view the website of the U.S. National Library of Medicine at: https://medlineplus.gov/druginfo/meds/a605022.html]. Clinical trials have shown that voriconazole is more effective and offers greater survival benefits compared with amphotericin B; making it a new standard therapy for invasive aspergillosis. There are safety issues with voriconazole as it has adverse side effects on vision and liver function, as well as a number of drug interactions. Posaconazole is effective against difficult to treat infections due to zygomycetes and ravuconazole has broad-spectrum potency in vitro and in vivo against a wide range of fungal pathogens (Boucher et al., 2004; Cecil & Wenzel, 2009).
Despite the advances offered by new drugs and new techniques the mortality associated with invasive fungal infections remains unacceptable, especially for individuals with severe immunosuppression as a result of chemotherapy, control of organ transplant rejection or HIV infection.
It remains the case that we have relatively few effective antifungal agents and resistance to them is a growing problem. Also, some patients do not respond to antifungal therapy despite being infected with fungi that show susceptibility to the drug in vitro, due to antifungal drug tolerance. Distinguishing tolerance from resistance might be important in avoiding failure of treatments (Berman & Krysan, 2020). Consequently, there is an undiminished need to find new antifungal agents. The search for novel antifungal targets has intensified since the establishment of several partial and complete genome sequences. Gene disruption in fungi can be particularly helpful as gene knockouts can identify essential genes, and a major effort is underway to discover novel antifungal targets for drug screening. These procedures are described in more detail below (Section 18.13).
Despite the need for new antifungals to cope with the growing need to treat systemic mycoses, most of the steps in the ergosterol biosynthetic pathway have not been exploited for development of antifungal compounds. Identification of specific enzyme inhibitors is technically difficult on the large scale required in drug discovery programmes because the enzymes are membrane bound and the substrates are difficult to handle. Whole cell screening methods have been developed and have shown promise in identifying specific, non-azole, inhibitors of enzymes in the ergosterol biosynthesis pathway.
The structure of the cell wall is unique to the fungi so there are no homologues in the human genome to any of the steps involved in fungal cell wall biosynthesis, yet only the echinocandins (Fig. 18.13) currently target the fungal cell wall by inhibiting the glucan synthase complex. There are other potential targets (Bowman & Free, 2003; Tada et al., 2013; Gow et al., 2017; Mazu et al., 2017):
- the chitin synthase complex; nikkomycins and polyoxins are useful competitive inhibitors, but there must be scope for antifungal agents that affect chitin synthase as noncompetitive inhibitors (analogous to the way echinocandins affect glucan synthase).
- Mannosyltransferases and glycosyltransferases used in the Golgi apparatus to synthesise the N-linked and O-linked oligosaccharides attached to cell wall glycoproteins represent a third target for antifungal drugs because it is known that fungal mutants defective in the biosynthesis of these oligosaccharides are severely affected in both growth and pathogenicity.
- The fourth target that could be exploited for the development of antifungal drugs is the process of generating and attaching a GPI anchor to cell wall proteins. Mutations that affect GPI anchor biosynthesis and attachment are lethal, demonstrating the importance of GPI-anchoring in the fungal cell wall. GPI anchor core structure is conserved between humans and fungi but there are differences in the number and placement of sugar residues onto the core structure that would provide fungal-specific steps in GPI anchor biosynthesis suitable for specific targeting.
- Enzymes that operate in the extracellular space (various cell wall glycosyltransferases) to assemble and cross-link cell wall components may offer the best cell wall target for an antifungal drugs because they would not have to cross the plasma membrane to get to their site of action and yet would target a critical step in cell wall biosynthesis. The antibacterial penicillins function in a very similar way to inhibit the cross-linking of bacterial cell wall components.
Currently the most promising of the approaches employed in clinical practice is combinatorial antifungal therapy which uses antifungal agents with different targets and spectra of activities in combination. Such treatment is expected to broaden antifungal coverage; become more fungicidal because of synergism between the antifungal agents used; minimise the risk of development of resistance; and lastly decrease toxicity. Unfortunately, cumulative evidence supporting the use of combinatorial antifungal therapy is conflicting and controversial. The value of using amphotericin B with flucytosine to reduce mortality in cryptococcal infections has been effectively proved, though in many developing countries flucytosine is not available and less effective combinations must be used.
The effectiveness of combinatorial antifungal therapy in the treatment of invasive fungal infections, like aspergillosis, is less clear. Very often, treatment outcomes are influenced more by the debilitation of the patient by their underlying condition rather than the combinations of antifungal treatments selected. In many cases combinatorial antifungal therapy is an option of last resort in fungal infections that have already shown high intrinsic resistance to current antifungal agents. Further, the patients who develop these invasive fungal infections are usually immunocompromised and because of this have a history of adverse factors that will affect their clinical responses to treatment of their fungal infections. For invasive aspergillosis, combination of voriconazole and anidulafungin has been shown to reduce mortality, so combinatorial antifungal therapy remains a potential potent option for management of invasive candidiasis, invasive aspergillosis, and rare mould infections (Johnson & Perfect, 2010; Garbati et al., 2012; Belanger et al., 2015).
An alternative combinatorial approach is significant and interesting because it illustrates the utility of detailed molecular knowledge well beyond the immediate focus of the drug and its immediate target to develop new methods of treatment based on entirely new concepts. This approach exploits the cellular role of the heat shock protein 90 (Hsp90), which is a molecular chaperone responsible for folding and maturation of client proteins (Panaretou & Zhai, 2008). Client proteins that acquire destabilising mutations can in some cases be stabilised by the molecular chaperone. This makes their activity dependent on Hsp90 activity. But the result is that Hsp90 can allow genetic (that is, mutational) variation to accumulate in a silent state that is revealed only when stress conditions overwhelm Hsp90. In the context of our present discussion ‘overwhelming stress’ can be taken to mean application of clinical antifungal agent, and Hsp90 certainly enhances the emergence of antifungal resistance; but the question is: would inhibition of Hsp90 reduce resistance to antifungal agents. The answer is yes: inhibition of Hsp90 reduces resistance of Candida albicans to azoles and enhances the effectiveness of the echinocandin caspofungin against Aspergillus species (Cowen, 2008; Semighini & Heitman, 2009).
In yeast and many other organisms, Hsp90 is expressed in excess of the level required for normal growth, which means that there is plenty of it available to buffer genetic variation and, consequently, influence evolution of the organism and its response to selection. This is biologically interesting but the effect of Hsp90 on emergence of resistance to antifungal agents suggests that Hsp90 could be a novel antifungal target. Several Hsp90 inhibitors (particularly geldanamycin and its analogues) have been combined with antifungal drugs with different modes of action against different fungi in vitro and in animal pathogenicity models. Geldanamycin is a 1,4-benzoquinone ansamycin antitumor antibiotic (a family of actinomycete secondary metabolites) that inhibits the function of Hsp90 by binding to an unusual ADP/ATP-binding site on the protein.
In all scenarios tested, inhibition of Hsp90 improved the response to antifungal drugs. Inhibitors of Hsp90 converted azole fungistatic activity against Candida albicans into fungicidal activity and enabled clearance of the fungal infection with fluconazole treatment. Similar effects were observed when Aspergillus fumigatus was treated with the combination of geldanamycin and caspofungin both in vitro and in an animal model. A similar strategy is to use genetically recombinant antibody targeted against fungal Hsp90 combined with an antifungal drug for treating life-threatening fungal infections (Burnie et al., 2006). Such findings support inhibition of Hsp90 in combination with fungistatic agents as a novel, and effective broad-spectrum therapy against fungal pathogens.
Because of the way that Hsp90 enhances emergence of antifungal resistance (that is, the evolution of resistance), inhibiting Hsp90 early in the infection has the potential to restrain the range of variation from which resistant isolates might be selected. In Darwinian terms, the ability to produce enough variation to allow adaptive evolution to occur is called evolvability. The idea that the evolvability of antifungal resistance can be blocked during treatment is an entirely new concept for a therapeutic approach (Cowen, 2008; Semighini & Heitman, 2009; Li et al., 2015; Lamoth et al., 2016).
Resources Box A Short History of Fungicides by Vince Morton & Theodor Staub (both formerly of Novartis Crop Protection, now Syngenta). CLICK HERE to download as a PDF This is an American Phytopathological Society Feature from March 2008 Compendium of Pesticide Common Names Alan Wood (formerly of CABI) has a website which is well worth a visit at this URL: http://www.alanwood.net/index.html that features a Compendium of Pesticide Common Names and chemical descriptions at http://www.alanwood.net/pesticides/index.html. |
Updated September, 2020
