9.8 Globose structures: sclerotia, stromata, ascomata and basidiomata
Sclerotia are pseudoparenchymatous hyphal aggregations in which concentric zones of tissue form an outer rind and inner medulla, with a cortex sometimes distinguishable between them. Sclerotia are tuber-like and detach from their parental mycelium at maturity. Some sclerotia consist of very few cells and are therefore of microscopic dimensions. At the other extreme, the sclerotium of Polyporus mylittae, found in the deserts of Australia, can reach 20-35 cm in diameter.
Sclerotia are stress-resistant, mycelial survival structures which pass through a period of dormancy before utilising their own accumulated reserves to ‘germinate’ (Erental et al., 2008). Dormant sclerotia may survive for several years, thanks to a rind composed of tightly-packed hyphal tips which become thick-walled and pigmented (melanised) to form an impervious surface layer. Such a layer of impervious tissue, which is especially protective against desiccation, may be developed in other circumstances to form what has been called a pseudosclerotial plate, for example, over the surface of hyphal structures of the fungus Hymenochaete corrugata binding hazel branches together and in similar adhesions between rhizomorphs and leaf litter in tropical forests. When such plates completely enclose the mass on which they are formed, the structure which results is called a sclerotium. Many fungi enclose portions of the substrate and/or substratum (which may include host cells if the fungus is a pathogen) within a layer of pigmented, thick-walled cells; the whole structure may be regarded as a kind of sclerotium.
The term sclerotium is, therefore, a functional one that encompasses a variety of structures. The different forms arose by convergent evolution, perhaps more than 14 times during fungal evolution (Smith et al., 2015), and most probably evolving, in Ascomycota, from aborted spore forming organs like perithecia, cleistothecia and conidiomata. In Basidiomycota there is some evidence for the same genes being involved in both sclerotium and fruit body initiation (in Coprinopsis cinerea; Moore, 1981), indicating that a common initiation pathway gives rise to hyphal aggregations which, under one set of environmental conditions (22-26ºC plus illumination), develop axial symmetry and become fruit body initials, but under other conditions (30-37ºC plus continuous darkness) develop radial symmetry and become sclerotia. Interestingly, analysis of the transcriptome and RNA editome (the spectrum of post-transcriptional RNA editing events during differentiation) during the early differentiation processes from vegetative mycelium to fruit body, oidia or sclerotia, confirms that the differentiation along all four of these developmental paths in C. cinerea is regulated transcriptionally and post-transcriptionally. Also, evolutionary analysis of the transcriptomes shows that formation of oidia has the lowest value of transcriptome age index (TAI). TAI quantifies the mean evolutionary age of a transcriptome; the lower the TAI, the older that part of the transcriptome in evolutionary terms. Transcriptomes of sclerotia and fruit body formation express evolutionarily younger genes (Xie et al., 2019).
The medulla forms the bulk of the sclerotium, and its cells (and those of the cortex where present) may accumulate reserves of glycogen and other polysaccharides, polyphosphate, protein and lipid. Sclerotium development comprises initiation, when the hyphae begin to aggregate to form small, distinct initials; development, when the initials expand and grow to full size, accumulating nutritional reserves from the parent mycelium; and maturation, which is most obviously characterised by clear demarcation of the surface and pigmentation of its constituent cell walls, but which also involves conversion of the reserve nutrients to forms suitable for long-term storage.
Sclerotia may germinate to form mycelium, conidia, ascomata or basidiomata, and the mode of germination seems in some cases to be a matter of size; small sclerotia germinate to produce a mycelium, large ones produce spores and fruit bodies. The giant sclerotium of Polyporus mylittae can form a basidioma without being supplied with water as the flesh is honeycombed with blocks of translucent tissue where the hyphae form copious amounts of an extrahyphal gel which serves as both nutrient and water store.
In many Ascomycota and Basidiomycota hyphae may aggregate to form fruiting structures which are responsible for producing and, just as important, distributing sexual spores. In Ascomycota, the sexually produced ascospores are contained in asci (singular: ascus) (shown in Fig. 5 of Chapter 8; CLICK HERE to view now) enclosed in an aggregation of hyphae termed an ascoma. Ascomata are formed from non-dikaryotic sterile hyphae surrounding the ascogonial hyphae of the centrum; a number of distinct types can be recognised (Fig. 15), including the open cup-like apothecia of Aleuria, the flask-like perithecium (found, for example in Neurospora and Sordaria) and the completely closed cleistothecium formed by, for example, Aspergillus (CLICK HERE for reminder). A similarly extensive range of diversity is found in ascospore shape and wall architecture; and ascus morphology, which may be globose or linear, and operculate (with a lid) or inoperculate (with an unlidded pore).
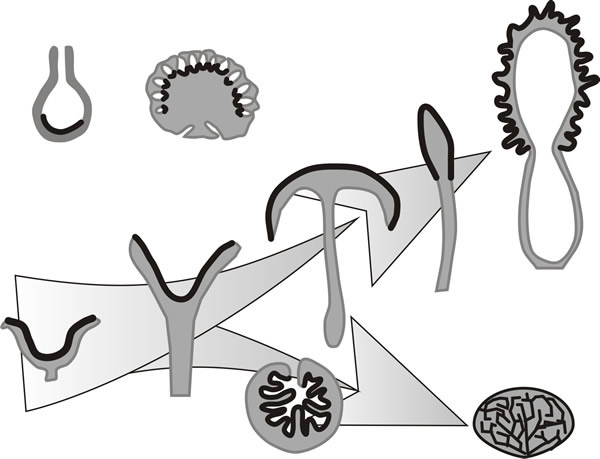 |
Fig. 15. The variety of multicellular fruiting bodies
of ascomycete fungi in the form of simplified diagrammatic sectional
drawings (redrawn after Burnett, 1968); in each case the hymenial tissue
is represented by the black line. The panel shows a range of ascomata
with, at top left a basic flask-like perithecium (e.g. Sordaria)
alongside the perithecial stroma of Daldinia. The rest of the
drawings are arranged to show how the simple cup-like ascoma of
Peziza might, hypothetically, have given rise, on the one hand, to
the morel (Morchella) via the fruit body forms of
Sarcoscypha, Helvella, and Mitrula, and on the
other hand to the subterranean fruit body of Tuber via a form
like Genea. Modified from Moore, 1995. |
The fruit-bodies of Basidiomycota, the mushrooms, toadstools, bracket fungi, puff-balls, stinkhorns, bird's nest fungi, etc., are all examples of basidiomata (singular: basidioma) which bear the sexually produced basidiospores on basidia (shown in Fig. 5 of Chapter 8; CLICK HERE to view now). Simplified diagrammatic drawings of some of the different types of basidiomata are shown in Fig 16. Again, an extensive range of diversity is found in basidiospore shape and wall architecture, and basidium morphology, and the shape and form of sterile accessory cells like cystidia and paraphyses.
The basidium is the meiocyte within which meiosis occurs and from which basidiospores develop (shown in Fig. 5 of Chapter 8; CLICK HERE to view now). Basidiospore are exospores (formed beyond the body of the meiocytes), ascospores are endospores (formed within the body of the meiocytes)(shown in Fig. 5 of Chapter 8; CLICK HERE to view now). In boletes and polypores basidia and basidiospores are formed by a hymenium lining the inner surface of pores on the lower surface of the fruit body. In the agaric or mushroom fungi the lower surface of the fruit body is developed into a set of plates (the gills or lamellae) on which the hymenium is located. Both structures are strategies to increase the surface area available for the spore-bearing hymenial tissue. Relative to spore production over a flat surface, gills achieve a maximum 20-fold increase in surface area. Poroid configurations of the hymenium produce larger increases in surface area, relative to gills; depending on the size and spacing of the pores, a poroid cap has an 18-fold to a 45-fold larger hymenium area relative to a flat surface (Fischer & Money, 2010).
However, pores have less efficient packing densities of basidia than gills because the inward curvature limits how close neighbouring basidia can be located before their spores run the risk of interfering with each other’s dispersal. Hence, the packing density of basidia ‘per unit mass of fruit body tissue’ is inherently less for pores than for gills. On the other hand, the protection (particularly against desiccation) offered by pores is greater, and this strategy suits perennial fruit bodies. A single fruit body of a perennial bracket fungus can produce 10 million spores per minute continuously for periods of 5 to 6 months. These brackets tend to be large fruit bodies; for comparison, an agaric fruit body (something like Agaricus campestris) can release about 2 million spores per minute, however such a mushroom is unlikely to last more than two weeks.
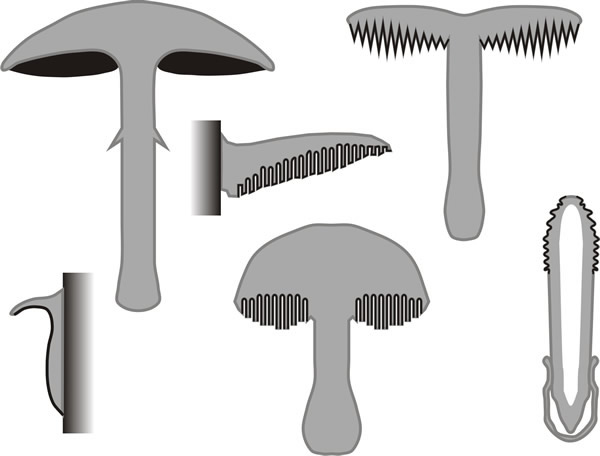 |
Fig. 16. The variety of multicellular fruiting bodies
of fungi in the form of simplified diagrammatic sectional drawings
(redrawn after Burnett, 1968); in each case the hymenial tissue is
represented by the black line. This panel shows a number of
basidiomata, including the agaric (mushroom) form of fruit body, the
poroid bracket and toadstool polypore, and toothed (hydnoid) form. At
bottom left is an encrusting fruit body (as in Stereum and
Phellinus) and at bottom right the mature stink-horn (Phallus).
From Moore, 1995. |
The spores are released from the under-surface of the cap and are distributed by air currents in the majority of species, so the cap must be supported above the substratum; this is the function of the stem. Basidiospores are violently discharged, and the four spores of a basidium are liberated within a few minutes of each other; many reproductive structures of terrestrial fungi are actively liberated using a wide range of mechanical processes for propulsion (Ingold, 1999). The basidiospore discharge mechanism is a violent (ballistic) discharge that clearly depends on the secretion of a droplet of fluid (water + solutes), which is called Buller’s drop, close to where the spore is joined to its supporting sterigma. A hydrophobic interaction with the spore surface is also probably important, generating what is known as a surface tension catapult (Turner & Webster, 1991; Money, 1998). This propels the spores directly away from the gill surface over distances ranging from 0.1 to 0.6 mm; spores with the largest Buller’s drop are propelled the greatest distance (Stolze-Rybczynski et al., 2009). During discharge these spores can be subjected to forces of acceleration several thousand times greater than that experienced by astronauts during the launch of rockets servicing the International Space Station (ISS)! Even more impressive is the fact that while those rockets consume 50% of their mass in fuel during the first 2 minutes of flight, ballistospore discharge is fuelled by metabolites that represent only 1% of the mass of the spore (Money, 1998). High-speed video analysis suggests that the size and shape of basidiospores affects discharge distance (Stolze-Rybczynski et al., 2009), because the asymmetric coalescence of Buller’s drop is what produces the launching momentum and launch direction (Liu et al., 2017). There is consequently a close linkage between spore size and morphology and the tube radii and distances between gills; and this close linkage is reflected in the evolution and adaptation of these aspects of the fruit bodies. Liu et al. (2017) describe ballistospore discharge as a generic catapulting mechanism for colloidal particles, and point out that understanding it has implications for many aspects of both biology and engineering.
The horizontal portion of the basidiospore flight path is halted by air viscosity within one millisecond of the launch and the spores then fall vertically until they clear the gills. Basidiospores are thus ‘shot’ into the space between adjacent gills, or towards the centre of the pore. They cannot be shot further than about the middle of that space to avoid being impacted onto the other gill (or the other side of the pore). Recent high-speed video analysis suggests that the size and shape of basidiospores affects discharge distance (Stolze-Rybczynski et al., 2009), probably by determining the size of Buller’s drop. There is consequently a close linkage between spore size and morphology and the tube radii and distances between gills; and this close linkage is reflected in the evolution and adaptation of these aspects of the fruit bodies.
Basidiospores rely on gravity to clear the gills or pores and emerge into the turbulent air beneath the fruit body, and this raises another point because the fruit bodies (and gills in many mushrooms) are exquisitely sensitive to gravity and can adjust growth differentials to bring a disturbed stem and gills back to the vertical. The shape changes which occur in agaric fruit bodies in response to change in the direction of gravity, usually referred to as gravitropism are morphogenetic changes.
When a mushroom fruit body growing vertically, as is normal, is disoriented (say, laid flat on the substratum by a foraging animal or person in a white coat) gravitropic bending results from differential growth of cells in the stem; those on the ‘bottom’ extend more than those on the ‘top’ so that the stem bends upwards. Calcium signalling is involved in regulating these growth differentials and proteomic analysis has revealed that at least fifty-one proteins are differentially-regulated during the response of Coprinopsis cinerea to gravity-disorientation (Kim et al., 2017; and see Häder, 2018). Gravity perception seems to be dependent on the actin cytoskeleton and nuclear motility; nuclei act as statoliths and interact with the actin cytoskeleton and trigger specific vesicle/microvacuole release from the endomembrane system to effect localised cell growth (Fig. 17; Moore et al., 1996).
The current interpretation is that this statolith-cytoskeleton interaction is communicated to the transmembrane domains of a sugar transporter located in the plasma membrane to change its substrate specificity and cause an otherwise uniformly distributed growth regulator (probably a laevorotatory 6-deoxy hexose sugar) to be asymmetrically transported into the disoriented cells (Moore & Novak Frazer, 2017; and see further discussion in Section 12.9). The function of all this precision engineering is to get the spores into the turbulent air beneath the cap so they can be distributed by the breeze. But it is another aspect of species diversity because the rate of perception and rate of reaction to changes in the physical environment of this sort are themselves species-specific.
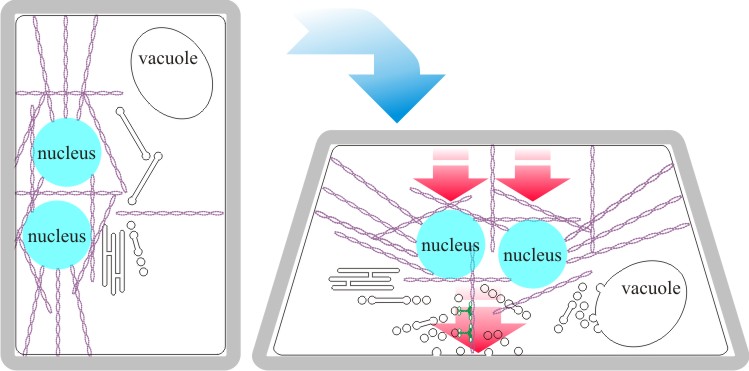 |
Fig. 17. Diagrammatic interpretation of gravity
perception in agarics. In Basidiomycota the two nuclei of a dikaryon are
enclosed in an F-actin cage in which they are held a particular distance
apart (the distance affects patterns of gene expression). Evidence
indicates that the nuclei act as statoliths to detect the gravitational
field and how this might work is illustrated here. In the vertical
orientation (normal for a fruit body stem, for example) the nuclei
within their actin cage are positioned within the cell by stressed
cables of actin microfilaments (on the left). If the orientation of the
tissue is disturbed (for example placed horizontal as on the right),
movement of the statoliths (nuclei) within the cage of actin changes the
stress on the actin cable connections to the endomembrane system and
generates useful directional signals. The current interpretation is
that this statolith-cytoskeleton interaction is communicated
to the transmembrane domains of a sugar transporter located in the
plasma membrane to change its substrate specificity and
cause an otherwise uniformly distributed growth regulator (probably a
laevorotatory 6-deoxy hexose sugar) to be asymmetrically transported
into the disoriented cells (Moore & Novak Frazer, 2017; and see further
discussion in
Section 12.9). Subsequently,
components required for wall modification and resynthesis will be
exported when the growth factor induces it. Because the wall resynthesis
is localised to the bottom wall, the cell will be curled upwards. This
effect, co-ordinated over many cells along the length of tissues like
fruit body stems, can reorient them to the vertical if they are
subjected to physical disturbance. Modified and redrawn after Moore
et al., 1996. |
Basidiomycota exhibit long-term plasmogamy because the ‘sexual’ (usually dikaryotic) mycelium has the ability to grow indefinitely. This is not so different from the indeterminate growth of heterokaryons in Ascomycota and it is clear that in both groups the sexually compatible mycelium can have a number of alternative developmental pathways open to it: continuation of hyphal growth, production of asexual spores, and progress into the sexual cycle (Moore, 1998). Choice between these is often a matter of the impact of very localised environmental conditions on a mycelium which has become competent to embark on a developmental pathway as a result of its capture and accumulation of nutritional resources. The flow chart in Fig. 18 shows an overview of the physiological processes involved in development of fruit bodies and other multicellular structures in fungi and gives some idea of the number of places where species-specific modifications can intervene in a general process to widen diversity.
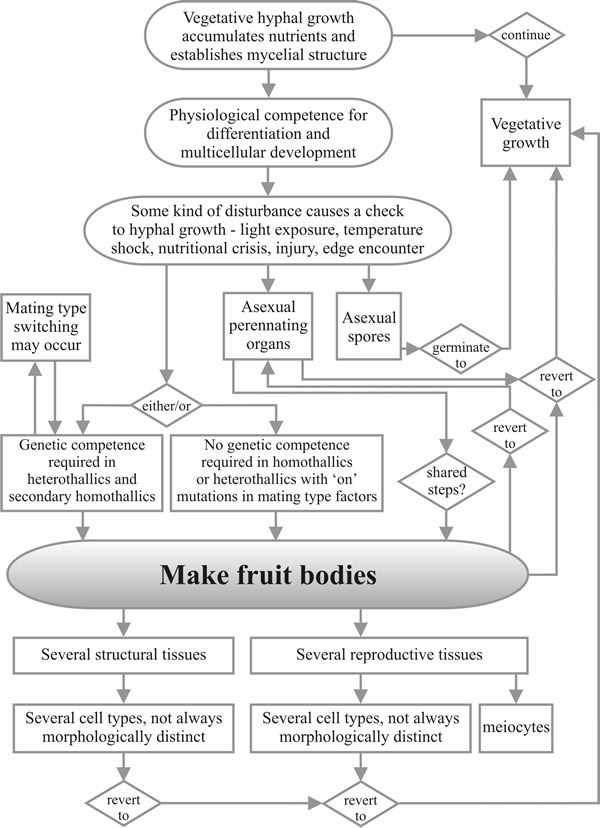 |
Fig. 18. Flow chart showing a summarised view of the
processes involved in development of fruit bodies and other
multicellular structures in fungi. Modified from Moore & Novak Frazer,
2002.
CLICK HERE to download a PDF version of this chart. |
Updated November, 2019
