8.4 Mating types of Neurospora
Species of Neurospora exhibit four different mating strategies:
- bipolar heterothallism with mating types A and a (in N. crassa, N. sitophila, N. intermedia and N. discreta) but mating type genes are present in a single copy per genome (unlike Saccharomyces cerevisiae);
- secondary homothallism (in N. tetrasperma) through the production of asci containing four ascospores each containing compatible nuclei;
- primary homothallism in which each haploid genome carries genetic information of both mating types (N. terricola, N. pannonica);
- primary homothallism, but in which genetic information for only one mating type can be detected (for example, N. africana possesses only an A idiomorph which shows 88% homology with the A idiomorph of N. crassa).
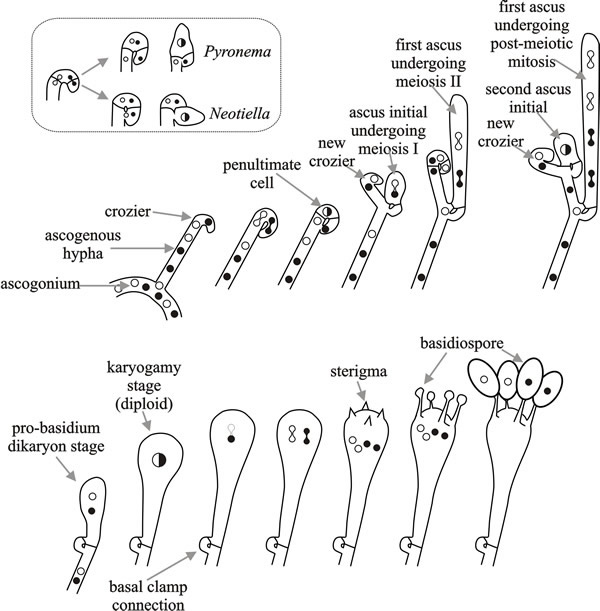
Species that show primary homothallism form linear eight-spored asci (octads) in which all progeny are self-fertile. In the bipolar heterothallic species, strains of both mating types develop ‘female’ structures (protoperithecia and their receptive hyphae, the trichogynes) under nitrogen starvation, as well as asexual spores (macroconidia or microconidia). A trichogyne of one mating type is attracted to and fuses with a cell of opposite mating type (a macroconidium, microconidium or hyphal fragment), which serves as the ‘male’ in a sexual cross. After fusion between the trichogyne and conidium, the ‘male’ nucleus is transported to the ascogonium at the base of the protoperithecium where several mitotic divisions occur.
Migration of (the still haploid) nuclei into the Neurospora crassa ascogonium (see Fig. 5) depends on mating type gene function and after their arrival there must be some mechanism to sort nuclei, to ensure that the subsequent meiosis only involves one a and one A nucleus, because mating type always segregates 1:1 in the progeny. It is likely that transient dikaryosis in the crozier involves a nuclear recognition mechanism. However, crozier abortion occurs regularly even in normal ascosporogenesis, so an alternative process might be that croziers that do not contain one a and one A nucleus are aborted.
Mating is followed by the formation of perithecia, within which as many as 200 asci are formed. Each ascus contains the products of a single meiosis. In many filamentous ascomycetes, a post-meiotic mitotic event, before ascospore formation, results in each ascus containing an octad comprised of four pairs of sister ascospores.
The first mating type genes to be cloned and sequenced in filamentous fungi were the A and a ‘alleles’ of Neurospora crassa (Fig. 6); these are idiomorphs of the mat locus. In all heterothallic filamentous ascomycetes examined to date, the mat locus is the sole determinant of mating type, and sexual reproduction is regulated by alternative mat A or mat a idiomorphs at this locus, which lack sequence similarity and encode different transcriptional regulators.
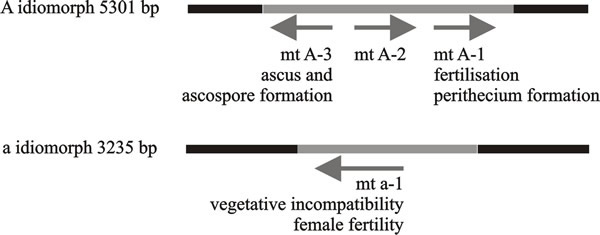 |
| Fig. 6. Functional regions of mating type factors of Neurospora crassa. The arrows indicate direction of transcription and the legends beneath the arrows indicate functions of the gene products. The black bars represent the conserved DNA sequences either side of the idiomorphs, which are shown as lines. These diagrams are oriented so that the centromere is on the left; consequently the centromere-distal sequence is on the right. Modified from Chapter 2 in Moore & Novak Frazer, 2002. |
In all the known Neurospora A and a idiomorphs the flanking regions are conserved, the region on the centromere side contains species-specific and/or mating type-specific DNA sequences. Immediately adjacent to these segments are regions that are very different between species. Next to these species-variable regions are the idiomorphs themselves. These are highly conserved between species but are completely dissimilar between the two mating types within the species. These are then followed by a ‘mating type common region’ of 57-69 bp, which separates an idiomorph from its nearby variable region and is very similar between species and between the two mating types.
The A idiomorph is 5,301 bp in length and gives rise to at least three transcripts (MAT A-1, A-2 and A-3), the first two being transcribed in the same direction (Fig. 6). The 85 amino acids at the N-terminal region of the mating type A product are the minimum required for expression of female fertility. The region from position 1 to 111 determines the vegetative incompatibility activity of the mating type locus, and amino acids from position 1 to 227 are required for male-mating activity. Mating type-specific mRNA is expressed constitutively in vegetative cultures, and continues to be expressed after mating, both before and after fertilisation. Transcript MAT A-1 is very similar to MATα1 of Saccharomyces cerevisiae and is essential for fertilisation and fruiting body formation.
The other two transcripts, MAT A-2 and MAT A-3, increase fertility and are essential in events after fertilisation, including ascus and ascospore formation, but are not essential for sexual development, which is controlled by MAT A-1. The MAT A-3 transcript has DNA binding ability and might function as a transcription-regulating factor. The function of the MAT A-2 polypeptide is not known. Its sequence contains motifs that occur in transcription activator proteins, but there are no obvious relatives in sequence databases other than the Podospora mating type homologue, SMR1.
The a idiomorph of Neurospora crassa has a single open reading frame (ORF) as the major mating regulator in mat a strains, which is 3235 base pairs long and gives rise to a single transcript (called MAT a-1) which encodes one polypeptide of 288 amino acids with DNA-binding activity. MAT a-1 is the only gene essential to mating in the a idiomorph, and has a role in the dikaryon stage. Amino acids 216-220 of the MAT a-1 polypeptide act in vegetative incompatibility while the region with DNA-binding activity is responsible for the mating function, implying that vegetative incompatibility and mating work through different mechanisms. The DNA sequences bound by MAT a-1 centre on CAAAG sequences, similar to ‘high mobility group’ (HMG) proteins that bind in the minor groove of the DNA helix and introduce a bend in the DNA molecule. DNA binding targets differ in different developmental stages, and the specificity may result from MAT a-1 interacting with unidentified protein factors. Mutations in either MAT a-1 or MAT A-1 cause mating defects.
N. crassa trichogynes respond to pheromones by bending towards the pheromone source in a mating type-specific way. Mating type mutants do not orient their growth towards pheromones. The pheromone receptor gene expressed in mat A cells, pre-1, is required for mat A trichogynes to recognise and fuse with mat a cells. The ccg-4 and mfa-1 pheromone genes are necessary for chemotropic attraction and ‘male’ fertility in mat A and mat a strains, respectively, so it is thought that pre-1 binds to mfa-1, while pre-2 recognises ccg-4. DNA sequences of fungal pheromone receptors predict a product with seven transmembrane segments with the ability to interact with a heterotrimeric G-protein linked to a protein kinase cascade. Transcription of pheromone receptor genes is regulated by the mating type factors (in Basidiomycota, pheromone receptors are products of a mating type locus, see below). A G-protein encoding gene with mating function, called gna-1, has been characterised in N. crassa. Female fertility is lost if gna-1 is disrupted. Female infertility also results if the gna-1 homologue in Cryphonectria parasitica, which is called cpg-1, is disrupted. This suggests that this G-protein is a component of a female pheromone response pathway that has been conserved in these filamentous ascomycetes. Pheromones and receptors are important for initial recognition of mates, but additional determinants, such as the presence of mat A and mat a genes in two different nuclei, are required for nuclear fusion, meiosis, and ascospore production (Kim et al. 2012).
Mating type genes evolve rapidly in Neurospora; the rapid divergence being due to either adaptive evolution or lack of selective constraints, depending on the reproductive mode of the taxa considered (Wik et al., 2008). Various mating type genes may also have alternative or additional functions during vegetative development of the mycelium. Mating type (mat) genes were increasingly expressed as the mycelium matured. This applies to both mating types though mat A more frequently exhibited a higher expression level than mat a, and pheromone and receptor genes were expressed in strains of both mating types in all development stages (Wang et al., 2012). Genes that respond to illumination showed similar expression timing during vegetative development in this study, so the mat genes may be part of the extremely complex light-controlled gene regulatory network in Neurospora crassa (Wu et al., 2014).
Updated July, 2019