4.5 Origin of drugs in current use: more about statins (contributed by Chee-Peng Lydia Pavey, 2003)
Abstract
Statins only became really important as a treatment for hyperlipidaemia within the last decade of the twentieth century. They are fast progressing as one of the most interesting and useful family of drugs available and are likely to become the 'wonder drugs' of the twenty-first century. There have been a number of clinically important trials performed on statins within the last ten years each allowing a greater understanding of CHD, its causes and risk factors. Not only can they be used to effective treat those at high risk of CHD, they have also been investigated in conjunction with the treatment of osteoporosis, multiple sclerosis, Alzheimer’s disease and in reducing the unwanted increased incidence in CHD in women on HRT treatment. Not everything is such a rosy picture however, as recently one of the major statins has been removed from the market followed reports of muscle wasting and deaths.
A brief introduction to statins
Coronary heart disease (CHD) is the leading cause of death in the world today and unfortunately this is a trend that looks set to stay as we become an aging population. In fact, one million Americans die from cardiac disease every year. Who, then, would have suspected that the humble fungi would be the answer to the physicians prayer with respect to tackling the epidemic of cardiovascular disease. From CHD (encompassing myocardial infarction (MI) and angina pectoris) to cerebrovascular disease to peripheral vascular disease, statins have proved to be the most effective and consistent treatment [1].
Aspergillus griseus is a soil-borne fungus. It produces secondary metabolites from which statins originate [2]. Strangely enough, fungal metabolites were initially looked at with respect to producing an antifungal agent but in fact were found to be far more adept at lowering cholesterol levels [3].
In the liver, cholesterol is synthesis from acyl coenzyme A (ACoA). ACoA relies on hydroxyl-β-methylglutaryl-coenzyme A (HMG-CoA) reductase to convert it to mevalonate and in turn this is converted to cholesterol. This step is the rate limiting step in the synthesis of cholesterol. Thus, if an HMG-CoA reductase inhibitor is introduced (which these fungal metabolites were found to be), then the amount of mevalonate will fall leading to a decrease in cholesterol which is needed for bile synthesis. This causes a compensatory upregulation in the number of LDL receptors on the cell surface and therefore a decrease in the amount of circulating LDL.
Statins are also able to reduce the hepatic production of VLDL and the amount of triglycerides circuiting in the blood, but the mechanism by which statins achieve this is unknown. There are also non-lipid effects of statins that are extremely important in the recent advancements in statin therapy that I shall be looking at later.
Little active compound reaches the circulation as statins are activated by first pass metabolism by the cytochrome P450 system yet deactivated by further metabolism. This is an important factor in drug interactions which I shall be looking at later.
Statins are well absorbed from the gut, yet have only a short half life and are mainly eliminated by the kidneys [4]. There are five main statins. Even though statins function via the same mechanism of action they are different in a number of ways [5].
| Table 1. Comparative features of statins [Reference 5] | ||||||
| simvastatin | pravastatin | fluvastatin | cerivastatin | atorvastatin | Comments | |
| Source | derived from Aspergillus | derived from Aspergillus | synthetic | synthetic | synthetic | simvastatin and pravastatin have similar chemical structures |
| Dose range | 10-80 mg | 20-40 mg | 20-80 mg | 300 microgram | 10-80 mg | |
| Effect on lipids
| TC 15-36% | TC 11-28% | TC 15-21% | TC 22% | TC 20-45% | atorvastatin - greatest effect on LDL and triglyceride |
| LDL 28-47% | LDL 19-35% | LDL 19-31% | LDL 30% | LDL 38-54% | ||
| HDL 5-13% | HDL 4-10% | HDL 2-10% | HDL 4-8% | HDL 3-7% | ||
| TG 1-36% | TG 4-24% | TG 1-12% | TG 17% | TG 16-46% | ||
| Renal excretion | 13% | 47% | 6% | 24-30% | <2% | renal impairment - adverse effects may be more frequent. Cerivastatin and pravastatin may need lower dosage |
| Hepatic metabolic isoenzymes
| CYP450 3A4 | CYP450 3A4 | CYP450 2C9 | CYP450 3A4 | CYP450 3A4 | most metabolised via same cytochrome P450 isoenzymes |
| CYP450 2C9 | CYP450 3C8 | |||||
| CYP450 2D6 | ||||||
| Proven prevention of coronary events | yes - in angina or history of myocardial infarction | yes - in patients with, or high risk populations without, prior myocardial infarction | no | no | no | long-term studies with clinical endpoints lacking for newer drugs |
| TC = total cholesterol, LDL = low density lipoprotein cholesterol, HDL = high density lipoprotein cholesterol, TG = triglyceride | ||||||
 |
 |
 |
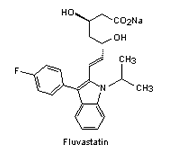 |
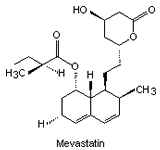
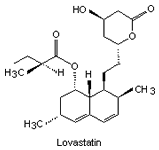
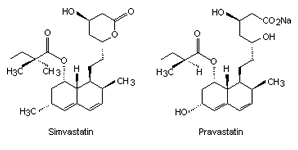
| Fig. 1. The structure of some of the statins [Ref. 6]. | |||
Important statin trials
The first major study investigating the treatment of hyperlipidaemia was the Scandinavian Simvastatin Survival Study (4S study). In effect, it acted as the catalyst for the following trials that I will also be looking at.
The 4S study took 4444 patients suffering from Ischemic Heart Disease (IHD) and elevated cholesterol levels (between 5.5-8 mmol/l) and gave them either simvastatin or a placebo. The patients were then closely monitored for a median of 5.4 years. During this period, a 25% decrease in the total cholesterol serum and a 35% decrease in the LDL cholesterol was observed [7]. Not only this, but the probability of a major coronary event occurring was reduced by 35%, that of coronary mortality by 30% and the need for coronary revascularisation with bypass surgery or an angiography was reduced by 37% [8]. This trial was important in the respect that it gave evidence highlighting that effective treatment of hyperlipidaemia with statins has a strong protective effect on people with IHD.
A year later, in 1995, the conclusions of the West of Scotland Coronary Prevention Study (WOSCOPS) were released. Now the emphasis was placed on primary prevention of CHD.
The WOSCOPS study took 6595 men aged between 35 and 64 year s of age. They were then given either 40 mg of pravastatin or a placebo and monitored for a mean of 4.9 years. Even though patients were chosen with no previous history of myocardial infarction [7], West Scotland has one of the highest IHD death rates in the world so it was therefore unsurprising that the men (gender itself being a risk category for CHD) picked for this study were all in a high risk group [5]: the mean cholesterol of the men was 7.03 mmol/l (± 0.57), the mean BMI was 26 (± 3.1kg/m² [ref. 7], 44% were current smokers, 13% were hypertensive, 5% had experienced angina and 8% had abnormal ECG tracings5. Results showed a 32% decrease in cardiovascular mortality, 37% decrease in revascularisation procedures [7] and a 31% reduction in myocardial infarction-caused or coronary artery disease (CAD)-caused death [5]. Even though the reduction in total mortality was 22% this only qualified as borderline significance. Even so, the results in general were indicative of the fact that people at high risk of CHD and who had not ‘yet’ become afflicted were suitable candidates for statin therapy.
Attention returned to that of secondary prevention of CHD and the year 1996 saw the publication of the ‘Cholesterol and Recurrent Events (CARE) Trial’.
This trial constituted 4159 patients who had previously experienced a myocardial infarction yet had average cholesterol levels i.e. 5.4mmol/l (± 0.4mmol/l). There was a median follow period of 5 years which yielded promising results: there was a 20% decrease in the level of total cholesterol, a 28% decrease in LDL cholesterol7, 31% reduction in the incident of stroke [8] (although this was only in cases where there was no previous history of stroke) [5] and most importantly a reduction of MI by 37% [8]. Thus, even though these post-MI patients had healthy cholesterol levels they still demonstrated the benefits of statin prescription [7]. That said, it must be noted that percentage reductions of 24% for coronary death and non fatal MI were relatively low compared to the 4S trial and consequently it appeared that patients with higher cholesterol levels reaped the benefits more [8].
The results of the CARE trial were verified by a further study conducted in 1998 called the ‘Long-term Intervention with pravastatin in ischemic disease' (LIPID) Study. Here, patients with history of MI but with varying cholesterol levels were given [7] 40mg of pravastatin. A greater number of participants (9014) took part and the follow up period was longer (a mean of 6.1 years) than the CARE study, but conclusions were the same [8], all patients benefited but those with higher cholesterol levels benefited the most.
Now that secondary prevention in CHD had been thoroughly investigated for the time being, primary prevention became the focus point of discussion and in 2001 results of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) were uncovered. This was a randomised trial on 5608 men and 999 women in which they took [7] 20-40mg of Lovaststin [8] for a mean of 5.2 years. The participants were all at average risk of CHD and had normal total cholesterol levels with the mean being 5.7 mmol/l (± 0.54) [7]. Again, the results showed dramatic decreases: primary end point (which represented fatal and non fatal MI, unstable angina and Sudden Cardiac Arrest) decreased by 37%, and the need for revascularisation was down by 33% [8]. But total mortality was virtually the same in both groups, of which two thirds were from noncardiovascular causes. Therefore one can conclude that statins, although capable of reducing the number of deaths from coronary causes, do not affect overall mortality rates as these average risk participants are just as likely to die from other causes. This is an important lesson in the considering the cost-effectiveness of statins and factors influencing prescription. This shall be addressed later [7].
The HPS study
It can undoubtedly be said that the Heart Protection Study (HPS) was one of the most ground-breaking trials concerning statins. Released in 2001 at the American Heart Scientific Session it is so far the largest and most far-reaching statin trial. 20,536 people at 69 different British Medical centres took part for a duration of 5 years [10]. Patients used were those who were at high risk of atheromatous disease because of previous coronary disease (i.e. these participant catered for the secondary prevention sector of the trial), those who had non-coronary atheromatous disease and those with diabetes [7]. Concerning other categories, e.g. serum cholesterol level and LDL cholesterol level and age etc., there were many variables thus adding to the multi-faceted nature of this trial [9]. The findings of the study are shown in Table 1.
| Table 1. Heart protection study primary outcomes | ||||
| Event | Simvastatin (n = 10,269) | Placebo (n = 10,267) | P | % Reduction |
| Death | 12.9% | 14.7% | .0003 | |
| Vascular causes | 7.6% | 9.1% | < .0007 | 17% |
| Any major vascular event | 19.8% | 25.2% | < .0001 | 24% |
| Coronary event | 8.7% | 11.8% | < .0001 | 27% |
| Non-fatal MI | 3.5% | 5.6% | < .0001 | 38% |
| Stroke | 4.3% | 5.7% | < .0001 | 25% |
| Revascularization | 9.1% |
11.7% |
< .0001 |
24% |
| Data from Reference 9. | ||||
Most important was the fact that the benefits, shown in the table above, were present to the same extent in all categories of people regardless of their LDL cholesterol level. This may appear at odds with the results of the CARE and LIPID studies, but we must remember that here all participants were high risk candidates for coronary heart disease (CHD) although their high risk may not have been due to LDL level but other possible risk factors. Potential risk factors for CHD are summarised in Table 2.
| Table 2. NCEP risk factors for coronary heart disease. |
Major positive CHD risk factors
Major negative CHD risk factors
Other positive CHD risk factors*
|
NCEP = National Cholesterol Education Program; CHD = coronary heart disease; LDL = low-density lipoprotein cholesterol; HDL = high-density lipoprotein cholesterol. *Other positive CHD risk factors are less well-proven or less acknowledged than the items listed under major positive risk factors. Data from Reference 10. |
Therefore the medical profession was presented with information that needed to be incorporated into new statin prescribing guidelines to best target those people who will benefit, even if their LDL cholesterol is not above the current mandatory ‘healthy’ level. Indeed, this will greatly increase the number of people who will be prescribed statins, but as things stand, the current guidelines are not being followed as it is, thus statins are greatly underutilised. But why is this?
One of the factors that may be discouraging the prescription of statins is the relatively recent withdrawal of Bayer’s statin drug ‘Baycol’ (Cerivastatin) in August 2001 by the manufacturer in agreement with the FDA [9]. This action was due to the increasing reports of serious myopathy and the reports of the serious, yet normally rare rhabdomyolysis [11] (a muscle wasting condition that can cause death). At the time the drug was withdrawn, more than 30 deaths had been reported [9].
There was an increased number of reports in conjunction with higher doses [11] (Baycol released a new high dose formulation, i.e. 0.8 mg of Cerivastatin in 2000)[9], in fact 60% of the cases fell under this parameter and many reported deaths were due to the co-prescription of Cerivastatin with gemfibrozil. Indeed the FDA enquiry confirmed that the incidence of fatal rhabdomyolysis was 16 to 80 time more frequent for Bayer’s Baycol than for any other statin, and even when the co-prescription of gemfibrozil was removed, the frequency was still 10 to 50 times higher.
All in all there have been more than 50,000 individuals taking part in statin trials, of which there have been no serious morbidity or increase in mortality. Statins are drugs with life-saving properties, they are the most effective drug for combating every aspect of atherosclerosis, are far more easy for the patient to cope with than a dramatic change in lifestyle and offer very few side effects (and we must remember that every drug has complications) [11].
Statins in postmenopausal women
The Women’s Health Initiative (WHI) had to halt an HRT trial after 5.2.years because the study was providing alarming results which highly suggested that HRT increased the incidence of many cardiovascular diseases. The overall cardiovascular disease incidence was increased by 22% , while the probability of stroke was increase by 41% and heart attacks by 29%. These findings were in keeping with those of the Heart and Eostrogen/progestin Replacement Study (HERS II) published only a week earlier.
It would not be viable to take women off their HRT treatment and it was decided that statin therapy would be ideal in reducing these increases cardiovascular related risks. Thus, after looking again at the HERS study but this time isolating those who were on a statin at the time procured the much needed results showing that these drugs reduced the risk of heart attack by 21%, the risk of all-cause mortality by 33% and the risk of venous thromboembolism by 55%. In fact, the incidence of a coronary event occurring within the first year of HRT prescription was reduced by 75% in those women co-prescribed with statins. Thus, the apparent major flaw of HRT had been effectively removed [9].
Statins and bone health
Osteoporosis occurs when skeletal loss by bone-reabsorbing osteoclasts exceeds that laid down by osteoblasts, the cells responsible for new bone formation. In 1999, Mundy et al [12] tested more than 30,000 drugs in the hope of finding one that would stimulate the production of a certain protein that is essential to bone formation (as it stimulates osteoblast differentiation) [13] called Bone Morphogenetic protein-2. Not all statins appear to have an effect on bone marrow the main factor appearing to be the uptake of the statin into the bone marrow. Lovastatin is proving particularly significant in its anabolic effects in the bone marrow [12] increasing the expression of BMP-2 RNA and more than doubling the amount of BMP-2 protein whereas fluvastatin, simvastatin and mevastatin showed less noticeable results.
Thus not only is the production of new bone increased but depressing the activity of the bone dissolving osteoclasts is also important. Therefore, statins have also attracted attention in conjunction with their mechanism in controlling hyperlipidaemia: as they are HMG-Co A inhibitors they inhibit the mevalonate pathway. G proteins which are essential in certain functions of osteoclasts require 20C and 15C side chains [12] (isoprenoids) which are added to them via a process known as prenylation. These side chains are produced through the very same mevalonate pathways that statins inhibit! Thus inhibition of these isoprenoids decreases the production of small GTPases, for example [13] Rho (involved in apoptosis and cytoskeletal organisation) and Rac (needed for endocytosis and membrane ruffling).
A study that looked at more than 1,200 patients in New Jersey aged 65 years and over found that there was a 70% decrease in the probability of a hip fracture in those currently using stains, a 50% decrease in those who had stopped using statins 6 months ago and a 45% reduction in those who had not used a statin for the past three years! Interestingly, oestrogen seemed to offer no protective effect from hip fractures.
A second study carried out by the United Kingdom General Practice Research Database, researching over 90,000 individuals, found the same trend with respect to the fact that statins reduced the incidence of fractures, but concluded that those who had not taken a statin for more than three months were not protected.
Of course, things are never so straight forward. In a double-blind study of pravastatin for atherosclerosis, those who developed fractures were noted and patients were grouped (unlike the UK General Practice research) according to a number of variables, among which were, body mass index, height, sex, age etc…This study failed to prove a beneficial link between statins and reduction in fracture incidence, but, it must be noted that pravastatin is water soluble and therefore is one of the least likely statins to be taken up by the bone in such quantities as to have an anabolic effect.
So far, these trials have failed to establish or discredit the protective effect of statins on bone and indeed have raised more questions:
- what are the different effects between different statins;
- if statins are protective, to what extent is this so;
- could statins ever replace the existing osteoporosis drugs [12].
Statins benefit the elderly
When looking at lipid-lowering drug prescriptions with respect to age a clear trend can be observed: the Odds Ratio (OR) for receiving a statin if one is between the ages of 55-64 is 1, this falls to 0.64 at ages 65-74 and to 0.16 at 75-84. But do these trends correlate with data on the effectiveness of statins in the elderly? [14]
The findings of the PROspective Study of pravastatin in the elderly at risk (PROSPER) trial was published online November 19th 2002 in The Lancet. This trial consisted of 2804 men and 3000 women aged between 70 to 82 years [15] who were at risk of vascular disease and of which some were randomly prescribed pravastatin at 40mg/d [8]. After an average follow up of 3.2 years [15] results showed that the LDL Cholesterol level had dropped by 34% and the primary end point (which was composed of coronary death, non-fatal MI and nonfatal/fatal cerebrovascular accident) decreased by 15%. Although the CAD attributed mortality rate was reduced by 24% there was no effect on all-cause mortality [8]. In a similar fashion as statin trials on the middle aged, the statins had a protective effect on CAD in as little as three years. These results were surprising in that the link between plasma cholesterol level and CAD decreases with age, yet these result show a definite benefit in the use of statins in the elderly, thus guidelines for vascular risk management should perhaps consider extending its focus beyond the middle aged to those in their 60 and 70s.
An important initial anomaly brought up by the PROSPER study was an increased incidence in cancer diagnosis in patients taking pravastatin. But, after a meta-analysis, there appeared to be no overall increased risk [15].
C-reactive protein and statins
For a long period of time now, it has been suggested but never really proved that inflammation has a key role in atherosclerosis. Also, that HDL –cholesterol has a protective effect on the risk of vascular disease, but yet again we are unsure as to how [16]. The important molecule at the centre of the inflammation quandary is C-reactive protein (CRP), it activates monocytes and is responsible for an increase in the production of cytokines [17].
Studies have shown that high levels of CRP increase the incidence of CHD, peripheral vascular disease, type 2 diabetes mellitus and stroke. Some of these trials even go on to suggest that CRP should be used as the primary measure of risk factor in CHD as not only is it an independent factor but it is also superior to other factors currently assessed (e.g. LDL cholesterol)!
A random cross-over comparison of 22 people compared the efficacy of three different statins in reducing CRP levels. Participants took either 40 mg of pravastatin, 20 mg of simvastatin or 10 mg of atorvastatin. After six weeks the reductions were 20.3%, 22.8% and 28.3% respectively. Coincidentally, these reductions were not related to changes in LDL cholesterol. This study has since been followed up with the recent publication of the ‘hsCRP and HDL Effects of Stain Trial’ (i.e. CHEST) in February 2003. This trial looked at the ability of a statin to reduce CRP levels and HDL levels and the time course and magnitude in which this occurs. Chest referred to the cross-over comparison study as inconclusive because of the small number of participants, the fact that people with diabetes were excluded and the actual cross-over design itself which did not allow for cRP levels to return to baseline in the washout phase.
In CHEST, 80 dyslipidemic adults without evidence of cardiovascular disease were prescribed either 10 mg of atorvastatin, 20 mg of simvastatin or 40 mg of pravastatin each day. After a 12 week course the reduction in CRP levels were 33%, 42% and 30% respectively and the decrease in LDL levels were 43%, 34% and 30%. In this case too, statins did not significantly reduce the level of HDL-cholesterol but the reduction in CRP levels directly correlated with that in LDL levels (Fig. 2).
 |
| Fig. 2. Relationship between log-transformed changes in CRP and LDL-cholesterol with statin treatment (correlation coefficient = 0.33, P = 0.004). [Reference 16] |
On longitudinal analysis the decrease in CRP levels over time appeared to be linear. As CRP levels did not level off over the 12 week period is can be concluded that further anti-inflammatory benefits may be observed in longer trials. Also, this progressive and consistent decrease in CRP levels indicates that the mechanism by which the statin reduces CRP levels must be indirect, as CRP has a very sort half life.
In 1998 the CARE study was reanalysed and it was found that those people who developed recurrent coronary events had CRP baselines at higher levels than those who did not. Interestingly enough, pravastatin did not conform to this statement and thus this points to its anti-inflammatory properties being the main factor for the reduction in these coronary incidents. Paradoxically, it was pravastatin that appeared to be briefly detrimental to decreasing CRP levels as in the first week of therapy, CRP levels actually increased, before conforming to the trend set by the other stains and decreasing the CRP levels steadily [16] (Fig. 3).
 |
| Fig. 3. Sample mean (bar height) and SD (error bar) CRP levels over time in substudy of 21 individuals receiving statin treatment. [Reference 16] |
So although CHEST did not answer many questions, as the findings differed from that of other studies it was useful in provoking more concise investigations including the mechanism by which statins reduced CRP levels and the link between CRP levels and LDL cholesterol [16].
Statins and multiple sclerosis
Multiple Sclerosis (MS) is an autoimmune disease. The immune system initiates inflammation in the perivascular white matter of the central nervous system. The blood-brain barrier is then breached by lymphocytes and macrophages which lead to demyelination of the nerves and the formation of plaques instead. Thus inflammatory cytokines aid this process and if there is too much destruction, the ability for myelin repair will be exceeded and scar tissue will predominate [4].
Scott Zamvil of the university of California, Sans Francisco [18] used simvastatin to treat mice with chronic and relapsing experimental autoimmune encephalomyelitis (EAE). EAE is a CD4+ Th-1 mediated CNS demyelinating disease that is frequently used as a model of MS [19]. After just a weeks treatment, which constitutes the highest does a patient would be offered [18], he histologically studied the brains and spinal chords of the mice and found a significant decrease in the number of perivascular lesions and extent of infiltration. On comparing in vitro samples taken from MS suffers treated with IFN beta-1b [20] (currently prescribed to reduced the inflammation process in acute attacks)[4] and those left untreated, statins demonstrated beneficial effects in both groups, thus suggesting that statins have a use as a co-prescriptive drug with pre-existing treatments, if not as a stand alone treatment [20].
Statins induce STAT 6 phosphorylation and secretion of Th-2 cytokines (IL-4, IL-5, IL-10) and transforming growth factor, yet decrease the secretion of Th1 cytokines (il-2, IL-12, interferon –γ) and tumour necrosis factor and inhibited STAT 4 phosphorlyation [19]. Thus, in all, promoting Th2 bias, which is considered protective against the disease [20].
Statins and Alzheimer's disease
At the 54th Annual Meeting of the American Academy of Neurology in April 2002, researchers from Boston University School of Medicine unveiled one of the biggest studies into the link between Alzheimer’s and statins yet performed. It consisted of 912 potential or definite patients with Alzheimer’s and 1669 family members who did not suffer from dementia. It was found that participants who took statins had a 79% decrease in the incidence of Alzheimer’s. This was the case even after factors such as age, race and apoE gene were taken into account [9]. The apolipoprotein E gene is situated on chromosome 19 and affects cell activity, it has three alleles, of which, the presence of ApoE e4 is associated with an increased risk of Alzheimer’s [21].
Conclusion
Hypolipidemic therapy has come such a long way over the past ten years that the field is virtually unrecognisable. This credit is all to be laid at the door of ‘the statins’. In the society in which we live today, the luxury of fine food and the excess of life often takes its toll on our cardiovascular system, hence it is vitally important that statins are utilised to as great efficacy as possible, both in this respect and as a possible opening for cures for various other diseases.
References
1. Singh, B.K. & Mehta, J.L. (2002). Management of dyslipidemia in the primary prevention of coronary heart disease. Current Opinion in Cardiology, 17: 503-511. URL: http://journals.lww.com/co-cardiology/Abstract/2002/09000/Management_of_dyslipidemia_in_the_primary.10.aspx.
2. Statins on the Patient.co.uk website. URL: http://www.patient.co.uk/health/Statins-(Cholesterol-Lowering-Medicines).htm.
3. Statins on Wikipedia. URL: http://en.wikipedia.org/wiki/Statin.
4. Waller, D.G., Renwick, A.G. & Hillier, K. (2001). Medical Pharmacology and Therapeutics. W.B. Saunders. pp. 457-460.
5. Hurley, E. (1999). Assessing the statins. Australian Prescriber, 22: 114-117. URL: http://www.australianprescriber.com/magazine/22/5/114/7.
6. Endo, A. (1992). The discovery and development of HMG-CoA reductase inhibitors. Journal of Lipid Research, 33: , 1569-1582.
7. Ong, H.T. (2002). Protecting the heart: a practical review of the statin studies. Medscape General Medicine 4, posted 12/10/2002. URL: http://www.medscape.com/viewarticle/445150_print.
8. Davidson, M.H. (2003). New tactics, new targets: the changing landscape of dyslipidemia management in coronary prevention. Medscape Education, published: 02/21/2003. URL: http://www.medscape.org/viewarticle/449790.
9. Del Negro, A. Does a statin a day keep the doctor away? Medscape Cardiology 6(2), posted 07/26/2002. URL: http://www.medscape.com/viewarticle/439009.
10. Crouch, M.A. (2001). Effective use of statins to prevent coronary heart disease. American Family Physician, 63: 309-321. URL: http://www.aafp.org/afp/2001/0115/p309.html.
11. Pasternak, R.C., Smith, S.C. Jr, Bairey-Merz, C.N., Grundy, S.M., Cleeman, J.I. & Lenfant, C. (2002). ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Journal of the American College of Cardiology, 40: 567-572. PDF
12. Mundy, G., Garrett, R., Harris, S., Chan, J., Chen, D., Rossini, G., Boyce, B., Zhao, M. & Gutierrez, G. (1999). Stimulation of bone formation in vitro and in rodents by statins. Science, 286: 1946-1949. DOI: http://dx.doi.org/10.1126/science.286.5446.1946. PDF
13. Katagiri, T., Yamaguchi, A., Komaki, M., Abe, E., Takahashi, N., Ikeda, T., Rosen, V., Wozney, J.M., Fujisawa-Sehara, A. & Suda, T. (1994).Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. The Journal of Cell Biology, 127: 1755-1766. PDF.
14. Watts, N. (2002). Bisphosphonates, statins, osteoporosis and atherosclerosis. Southern Medical Journal, 95: 578-582. URL: http://www.medscape.com/viewarticle/438222. PDF.
15. Rogers, M.J. (2000). Statins: lower lipids and better bones? Nature Medecine, 6: 21 - 23. DOI: http://dx.doi.org/10.1038/71484.
16. De Wilde, S., Carey I.M., Bremner, S.A., Richards, N., Hilton, S.R. & Cook, D.G. (2003). Evolution of statin prescribing 1994-2001: a case of ageism but not of sexism? Heart, 89: 417-421. DOI: http://dx.doi.org/10.1136/heart.89.4.417. PDF.
17. Barclay, L. ( 2002). Statins benefit the elderly as well as the middle-aged. Medscape Medical News, Nov. 19, 2002. URL: http://www.medscape.com/viewarticle/444791.
18. Ansell, B.J., Watson, K.E., Weiss, R.E. & Funarow, G.C. (2003). hsCRP and HDL effects of statins trial (CHEST): rapid effect of statin therapy on C-reactive protein and high-density lipoprotein levels: a clinical investigation. Heart Disease, 5: 2-7. PDF.
19. Ganong, W.F. (2001). Review of Medical Physiology. Twentieth International Edition. McGraw-Hill Inc. ISBN-10: 0071120645, ISBN-13: 978-0071120647.
20. Pearson, H. (2002). Heart drugs soothe brain inflammation. Nature News Published online 7 November 2002. DOI: http://dx.doi.org/10.1038/news021104-10. URL.
21. Youssef, S., Stüve, O., Patarroyo, J.C. , Ruiz, P.J., Radosevich, J.L., Hur, E.M., Bravo, M., Mitchell, D.J., Sobel, R.A., Steinman, L. & Zamvil, S.S. (2002). The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature, 420: 78-84. DOI: http://dx.doi.org/10.1038/nature01158.
22. Pozzilli, C. & Prosperini, L. (2010). Recent advances in the management of multiple sclerosis. European Neurological Journal, 2: 41-48. URL: http://www.slm-neurology.com/the-european-neurological-journal/details/article/recent-advances-in-the-management-of-multiple-sclerosis/. PDF.
23. Diamond, J. (2009). Alzheimer's disease: causes of Alzheimer's disease. The Alzheimer Society of Canada website. URL: http://www.alzheimer.ca/english/disease/causes-riskfac.htm. PDF.
Updated December 7, 2016
