14.13 Host penetration through stomatal openings
An appressorium is a highly organized enlarged end of a hypha. When over a stoma, a penetration peg is formed and enters directly through the stoma into the substomatal chamber. From here the fungal hyphae grow through the middle lamella between the cells of the leaf tissue, so they can extract the nutrients they require without killing the host tissue.
Host penetration through stomatal openings occurs in the rust fungi, Uromyces spp. and a specific example is Uromyces fabae (Basidiomycota), the bean rust (Voegele, 2006). U. fabae attacks several important crop species: broad bean (Vicia faba), pea (Pisum sativum), and lentil (Lens culinaris), as well as more than 50 other Vicia species, and about 20 Lathyrus species (sweet peas and grass peas). It is most important in causing rust disease of broad bean (faba bean) on which it can cause up to 50% yield losses. U. fabae is a macrocyclic rust fungus, having all five types of spore known in the Uredinales (listed in the bullet point below), and as all of its spores are formed on a single host it is also described as autoecious. The lifecycle of U. fabae is as follows:
- diploid teliospores that have overwintered on plant debris in the field germinate in spring, forming a metabasidium;
- meiosis occurs and the metabasidium forms four haploid basidiospores (of two different mating types);
- basidiospores are ejected from the metabasidium;
- after landing on a host leaf, basidiospores germinate and produce the infection structures;
- in the diseased host the pathogen mycelium produces pycnia containing pycniospores and receptive hyphae;
- pycniospores are exchanged between pycnia of different mating types and dikaryotisation occurs, forming aecial primordia;
- an aecium differentiates and forms dikaryotic aeciospores;
- aeciospores germinate on the host and form infection structures;
- uredia are produced by this dikaryotic infection, and these produce urediospores;
- urediospores are the major aerially dispersed asexual spore form, being produced in large numbers during the summer in repeated infections of host plants (urediospores are the ‘rust’ of rust fungi);
- at the end of summer, uredia differentiate into telia, nuclei fuse during sporogenesis to form the single-celled diploid teliospores that overwinter on the remains of the host.
Most information about rust infection structures derives from analysis of urediospores (Fig. 5). Urediospores (Fig. 5a) germinate with a germ tube which differentiates into a well defined appressorium that forms a penetration hypha which enters the leaf through the stomatal opening and expands into a vesicle in the stomatal cavity. An infection hypha grows out of the vesicle, and when this contacts a mesophyll cell it differentiates a haustorial mother cell from which a haustorium is formed to penetrate the host cell.
The penetration mechanism of germinating basidiospores is completely different, being a direct penetration (detailed below in the section on powdery mildews; CLICK HERE to view the page), although the appressorium, vesicle and haustorium infection structures are still formed (Fig. 5b) (Voegele, 2006).
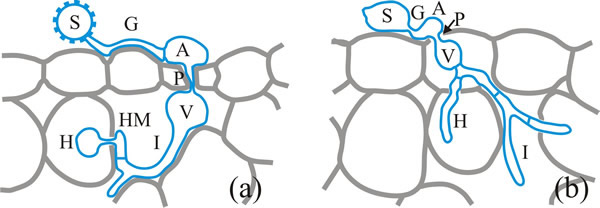 |
| Fig. 5. Infection structures derived from urediospores (a) and basidiospores (b) of the bean rust, Uromyces fabae. S, spore; G, germ tube; A, appressorium; P, penetration hypha; V, vesicle; I, infection hypha, HM, haustorial mother cell; H, haustorium. Modified from a drawing in Voegele, 2006. |
Urediospores are single-celled, and their walls have spiny, hydrophobic surfaces. Hydrophobic interactions are responsible for initial adhesion to the host surface. Initial contact with the host triggers production of an extracellular matrix comprising low molecular weight carbohydrates and glycosylated polypeptides that originates by lysis of the germ pore plug of the spore. This contributes to formation of an adhesion pad, to which fungal cutinases and esterases also contribute. A period of at least 40 minutes of darkness is required to induce germination and a temperature in the range 5 to 26°C (optimum 20°C), but urediospores germinate on almost any surface under these conditions so no signals from the host are needed to induce germination.
Fungal cytoplasm moves into the germ tube as it meanders across the surface to which it is attached by the adhesive extracellular matrix material. This is where the topographical signals to which we referred above come into effect. Differentiation of an appressorium in Uromyces appendiculatus and U. vignae is induced by a ridge 0.4 to 0.8 µm in height, which corresponds to the height of the stomatal guard cell lips. On induction, the cytoplasm transfers to the appressorium and the vacuolated germ tube is separated off by a septum. Differentiation of the appressorium coincides with the appearance of several (fungal) lytic enzymes (Fig. 6): cellulases, proteinases and chitin deacetylase.
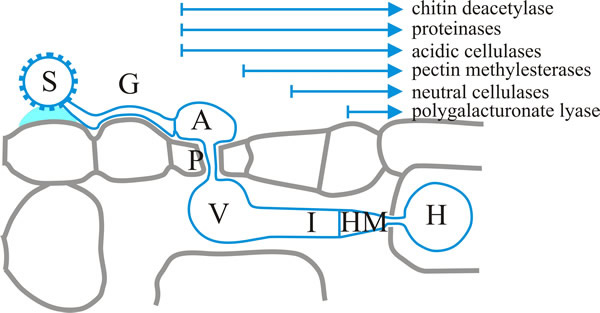 |
| Fig. 6. A succession of lytic enzyme production occurs during early dikaryotic infection structures of the bean rust, Uromyces fabae. Here the start of each arrow indicates upregulation of the enzymes noted during development of the infection structure from appressorium to haustorium. Key: S, spore; G, germ tube; A, appressorium; P, penetration hypha; V, vesicle; I, infection hypha; HM, haustorial mother cell; H, haustorium. Modified from a drawing in Voegele, 2006. |
A penetration hypha is formed from the base of the appressorium, extending into the stomatal cavity where a substomatal vesicle forms and is separated from the penetration hypha by a septum. The vesicle narrows into an infection hypha and more fungal enzymes appear, which contribute to localised breakdown of the host cell wall: pectin esterases and methylesterases, and cellulases (Fig. 6). A haustorial mother cell differentiates when the infection hypha contacts a mesophyll cell, and becomes separated from the infection hypha by a septum. The cytoplasm moves into the haustorial mother cell and earlier structures are vacuolated. Formation of the haustorial mother cell coincides with the onset of polygalacturonate lyase activity (Fig. 6) (Voegele, 2006).
All the infection structures so far described (which make up the process described as the penetration phase) can be generated in vitro by germinating spores on plastic membranes. Haustoria, and structures of the parasitic phase, and the later sporulation phase are only formed in plants (Fig. 7) because signals from the host are needed to complete differentiation of the haustorium.
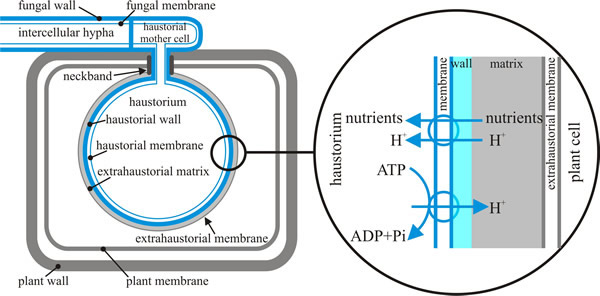 |
| Fig. 7. Schematic representation of a dikaryotic haustorium of the bean rust, Uromyces fabae within a mesophyll cell of its host, Vicia faba. This illustration is intended to emphasise the number and arrangement of the barriers (membranes and walls) between the pathogen fungus and its host plant. Structures derived from the fungus are depicted in blue; structures contributed by the plant are shown in grey, including the extrahaustorial matrix (though the fungus also contributes to the matrix) and the extrahaustorial membrane. The magnification shown at right shows how proton pumping by fungal ATPases powers uptake of nutrients from the plant cell by fungal symports. Based on illustrations in Voegele, 2006. |
Formation of the haustorium involves breeching the cell wall of the host cell and invagination of its plasma membrane. Although within the volume of the host cell, the haustorium remains outside the physiological boundary of the host being separated by the plasma membranes of parasite and host, the fungal cell wall and the extrahaustorial matrix. The latter is a mixture of carbohydrates and proteins, which mainly originates from the host plant and may be a symplastic compartment (the symplast is the inner side of the plasma membrane in which water and solutes freely diffuse) or a specialised part of the apoplast (the space outside the plasma membrane in which materials freely diffuse) (Voegele, 2006).
Updated July, 2019
