12.12 Developmental commitment
There is, then, a wealth of evidence for highly specific differentiation of individual cells in fungi. There is even some direct evidence for that developmentally-important concept: commitment. This is the process whereby a cell becomes firmly committed to just one of the several developmental pathways that are open to it before expressing the phenotype of the differentiated cell type. This has always been an important concept in metazoan development, and with the recent growth of interest in the potential of embryonic stem cells in medical treatments, it is still a major line of research. The research is intended to establish what factors endow an embryonic stem cell with the plasticity to give rise to several differentiated types of cell; what signals drive cells to commit to a particular cell fate; and whether the decision to differentiate along a particular developmental path is irreversible (Lassar & Orkin, 2001); all, of course, in the hope of using stem cells in disease therapies (Lassar & Orkin, 2001; Trounson & McDonald, 2015).
The classic demonstration of developmental commitment involves transplantation of the cell into a new environment; if the transplanted cell continues along the developmental pathway characteristic of its origin then it is said to have been committed prior to transplant. On the other hand, if the transplanted cell embarks upon the pathway appropriate to its new environment then it was clearly not committed at the time of transplant. Most fungal structures produce vegetative hyphae very readily when disturbed and ‘transplanted’ to a new ‘environment’ (which is usually an in vitro culture medium). Strictly, of course, this is a regenerative phenomenon, important in its own right and a very significant and experimentally attractive attribute of fungi. Yet it does create the impression that fungal cells express little commitment to their state of differentiation.
Very little formal transplantation experimentation has been reported with fungal multicellular structures. The clearest examples of commitment to a developmental pathway in fungi have been provided by Bastouill-Descollonges & Manachère (1984) and Chiu & Moore (1988a) who demonstrated that basidia of isolated gills of Coprinellus congregatus and Coprinopsis cinerea, respectively, continued development to spore production if removed to agar medium at early meiotic stages. Similar results were obtained whether water agar, buffered agar or nutrient agar was used as explantation medium. Other hymenial cells, cystidia, paraphyses and tramal cells, immediately reverted to hyphal growth but this did not often happen to immature basidia. Evidently, basidia are specified irreversibly as meiocytes and they become committed to complete the sporulation programme during meiotic prophase I.
Clearly, then, these experiments demonstrate commitment to the basidium differentiation (sporulation) pathway some time before the differentiated phenotype arises in these fungi. It is also important to stress that other cells of the hymenium do not show commitment. Rather, they immediately revert to hyphal growth on explantation as though they have an extremely tenuous grasp on their state of differentiation. That these cells do not default to hyphal growth in situ implies that some aspect of the environment of the tissue that they normally comprise somehow continually reinforces their state of differentiation. These non-committed cells must be considered to be totipotent stem cells. It is this uncommitted state of differentiation of most of the cells in mushroom fruit bodies that accounts for the readiness of field-collected mushrooms to revert to vegetative growth when fragments are inoculated to culture medium. Mycologists expect as a matter of common routine to be able to make cultures in this way, using the simplest media, from fruit bodies they collect. No animal or plant ecologist can expect to be able to do this. The difference denotes a significant quality of fungal cell differentiation.
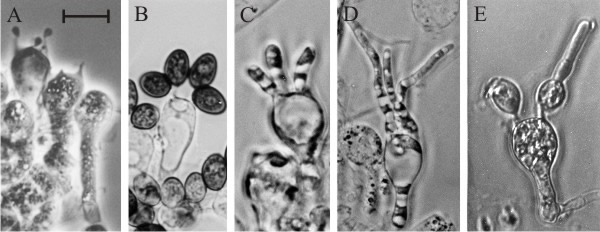 |
| Fig. 25. Basidium development of Coprinopsis cinerea is disrupted by application of ammonium salts. This can be demonstrated by in vitro experiments in which slivers of gill tissue cut from a developing fruit body are transferred to agar medium. Providing they are transplanted after prophase I of meiosis (A), Coprinopsis basidia are committed to continue development and they complete meiosis and spore formation after excision from the fruit body and transfer to water agar (B). If ammonium salts are included in the medium normal developmental is arrested; instead of forming sterigmata and spores, ammonium-treated basidia immediately dedifferentiate to produce vegetative hyphal tips (C, D and E) from regions of active wall growth. In C and D, the sterigmata have grown out as vegetative hyphal tips. In E the hyphal tip has grown from the top of an incomplete spore. Note that this is not equivalent to premature germination because the germ pore (from which a mature spore will germinate) is located close to the sterigma attachment point. In specimen E the ammonium treatment has caused the emergence of a hyphal tip from a region of primary wall growth in the incomplete spore. Scale bar = 10 µm. Photomicrographs by Prof. S.-W. Chiu, Chinese University of Hong Kong. Illustration modified from Moore (1998a). |
Protobasidia of Coprinopsis cinerea in the prekaryogamy (dikaryotic) stage at the time of excision of a transplant were arrested at that stage; explants made at later physiological ages did complete meiosis and/or sporulation, though at a slower rate than in vivo. Once initiated, the maturation of basidia is an autonomous, endotrophic process which is able to proceed in vitro; but it can be inhibited. Post-prophase I protobasidia were committed to the meiocyte programme of differentiation through meiosis and culminating in basidiospore maturation even when explanted to water agar. This in vitro explantation process has been developed as a rapid, small‑scale bioassay for chemicals which interfere with sporulation processes. Simply including various compounds in the explantation medium inhibited basidium differentiation. Growth is not inhibited in the bioassay; rather, differentiation inhibitors cause vegetative hyphal tips to grow out from regions of the basidial apparatus expected to be in active growth during sporulation (Fig. 25) (Chiu & Moore, 1988b, 1990c).
The differentiation inhibitors tested fell into two groups:
- ammonium salts and immediate structural analogues, including alkali metal salts, and glutamine, and some of its structural analogues were effective in tissues exposed at any time after meiotic division I (even into post-meiotic sporulation stages);
- ionophores, cAMP and wall synthesis inhibitors were effective only if applied during meiosis.
Differential sensitivity of basidia between meiotic and sporulation stages implies that during the nuclear division the cell is prepared in advance for sporulation so that by the end of the cytologically recognisable nuclear division sporulation can proceed despite the metabolic disturbance caused by ionophores and wall synthesis inhibitors. On the other hand, the sporulation process must involve essential components that are sensitive to excess ammonium (and glutamine, etc) but which remain crucial after completion of the nuclear division. Depending on the stage reached at the time of exposure to the inhibitors, vegetative hyphal tips emerged from the four apical sites for sterigmata, from the tips of sterigmata, from partially formed or abnormal spores, and from the basal regions of the basidium from which paraphyses would be expected to arise. These and other data allow basidium differentiation in Coprinopsis cinerea to be categorised into a sequence of steps of commitment comprising:
- commitment to recombination;
- commitment to meiosis;
- commitment to sporulation.
The ability of exogenous ammonium and glutamine to inhibit sporulation and promote vegetative growth in these explantation experiments is complementary to the observations described in the previous section, that ammonium‑scavenging NADP-GDH and GS activities are naturally increased in fruit body caps as the fruit body matures (CLICK HERE to view a reminder) and allow completion of the cycle of interpretations.
We can infer from the observations and experiments that within the normally-developing C. cinerea fruit body ammonium‑scavenging enzymes are specifically derepressed to prevent ammonium ions halting meiocyte differentiation and terminating sporulation. C. cinerea grows naturally in compost where ammonia is abundant and volatile, so the ammonium assimilating enzymes are produced in the cap to serve as an efficient ammonium-scavenging system that maintains the microenvironment surrounding the hymenium free from the inhibitor. The proof of this seems to be that when the system was overloaded by injecting solutions of ammonium salts into the cap, the meiotic pathway was halted, leaving white patches of non-sporing hymenium on the mature cap (Chiu & Moore, 1990c). Thus, meiosis and sporulation can be shown to be sensitive to inhibition by ammonium ions in vivo and in vitro, and to have a natural defence system built into its developmental programme
Updated July, 2019
