4.11 Hyphal branching
Growth of the mycelium depends on formation of hyphal branches. To proliferate a hypha must branch. There is no other way to turn one hypha into two hyphae.
Although apical (or near-apical) branching of fungal hyphae does occur, most branches are laterally placed. Hyphal branches may be described as primary (which subtend no branches and arise directly from the main hypha), secondary (which subtend a primary branch), tertiary (which subtend a secondary branch), etc. In many biological and non-biological branching systems there is an inverse logarithmic relation between the number of branches belonging to a particular order and order number; a relationship that allows maximum surface area to be colonised by minimum total length of filament. The relation holds in the majority of fungi and reflects the efficiency of the mycelium in colonising the substratum while minimising the amount of biomass required to do so.
In early phases of growth, branches usually subtend an angle of approximately 90° to the long axis of the parent hypha. As we have seen, hyphae tend to avoid their neighbours (negative autotropism) and to grow radially away from the centre of the colony. So a circular colony is formed eventually, with radially directed hyphae, approximately equally spaced, and extending at the margin at a constant rate.
As the colony circumference increases the apices of some branches catch up with their parent hyphae to maintain hyphal spacing at the colony margin. This occurs either by relaxation of controls on extension rate of branches as they become further separated from main hyphae or as a result of simple variability in extension rate.
A specific example of change in hyphal behaviour during colony development is provided by an analysis of mycelial differentiation in Neurospora crassa (McLean & Prosser, 1987). Up to about 20 hours of growth, all hyphae in mycelia of this fungus have similar diameters, growth zone lengths and extension rates and all branches are at an angle of 90° to the parent hypha. After about 22 hours growth branch angle decreases to 63°, hyphal extension rates and diameters increase and a hierarchy is established in which main hyphae are wider and have greater extension rates than their branches; the ratios between diameters of leading hyphae, primary branches and secondary branches being 100:66:42 and between extension rates being 100:62:26.
For a lateral branch to emerge from a region of a hypha with a mature rigidified wall requires the (internal) assembly of a new hyphal apex at the site of emergence. What specifies the site of the branch initiation is not known yet. Although a branch can potentially form at any point on a hyphal wall, in fungi that form septa there is usually a close relationship between septation and branching; branches may form at a specific time after septation and be positioned immediately behind septa.
In most fungi, though, the septation/branching relationship is less obvious and branch positioning is more variable. Indeed, although zygomycetes follow the same growth and branching kinetics as Ascomycota and Basidiomycota, only young mycelia of Mucor hiemalis and Mucor rammanianus form septa, mature zygomycete mycelia only form septa at the base of sporangia so in lead hyphae there is usually no relationship between septation and branching.
At one time, localised fluxes of ions through the hyphal membrane were thought to be involved in determining branching. Application of electrical fields certainly affects the site of branch formation and the direction of hyphal growth in young mycelia of several fungi, but endogenous ion fluxes seem to be more related to nutrient uptake than tip growth.
Several compounds act as paramorphogens, inhibiting hyphal extension and increasing hyphal branching. The (long) list includes non-metabolised sugar analogues (like L-sorbose, which is a hexose analogue, and validamycin A, which is a pseudo-oligosaccharide), inhibitors of phosphoinositide turnover, cyclic-AMP, and cyclosporin (Fig. 21), but the wide range of cellular targets represented does not help in understanding how branches initiate.
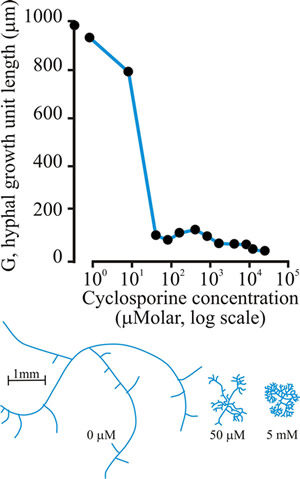 |
Fig. 21. Effect of cyclosporin-A on the hyphal growth unit (shown graphically at top), and the morphology of a wild type strain of Neurospora crassa grown at 25°C on a solid glucose-minimal salts medium (illustrated in the habit sketches beneath the graph, which also indicate the concentration of cyclosporin that causes the morphology shown). Modified and redrawn after Trinci, Wiebe & Robson, 1994. |
One interesting possibility is that heat-shock proteins may be involved in the mechanism for branch initiation. Heat-shock proteins are polypeptides which interact with other proteins. They are ‘molecular chaperones’ which bind to and stabilise other proteins to prevent incorrect intermolecular associations, then aid their correct folding by releasing them in a controlled manner. It is feasible that branch initiation requires assisted conformational alterations of wall proteins or that heat-shock proteins assist in the delivery of a branch-initiating polypeptide to the appropriate position.
Updated July, 2019
