4. A fungus-like root for the eukaryote tree
Martin et al. (2003, p. 197) suggested that a eukaryotic phylogenetic tree with fungi first would make sense. These authors based their overall tree of life on the standard three-domain model and showed the stem eukaryotes as emerging from within the archaebacteria. I would adhere, as outlined above, to the four ages of life as set out by Cavalier-Smith (2010a) but would start the age of eukaryotes about 1.5 – 2 billion years ago and amend the origin of eukaryotes as follows (Fig. 3).
The eukaryotic stem added the mitochondrion by enslavement of a bacterium (and perhaps added the nucleus by enslavement of an archaean, depending on the timing of divergences of prokaryote groups), and later evolved the endomembrane system and cytoskeletal architecture. The following features, which in the present day are characteristics of fungi, emerged in this temporal sequence:
- Free cell formation the cytoskeletal organisation to manage vesicle and organelle trafficking and particularly the positioning of wall- and membrane-forming vesicles to enclose volumes of cytoplasm to subdivide sporangia into spores (see discussion in Moore et al., 2011, pp. 48-50), with adoption of a chitinous cell wall, possibly as an adaptation of the ancestral actinobacterial mechanism for addition of oligosaccharides containing N-acetylglucosamine to surface proteins (muramopeptide wall precursors).
- After this process is established, this is a potential branch point for divergence at the unicellular level to plants and heterokonts, with phragmoplast formation left as a vestige of free cell formation specifically localised at the division spindle equator, and the early cell wall adapted to be a polymer of glucose rather than N-acetylglucosamine, possibly to economise on the demand for reduced nitrogen in an organism that is abandoning heterotrophy in favour of photosynthesis after enslavement of a cyanobacterium.
- Filamentous growth first to make rhizoids and sporangial necks and stalks in chytrid-like opisthokont cells. Limiting wall growth to an apex even in an opisthokont cell involves creation of a coordinated production and distribution system for wall and membrane precursors and enzymes; together with a cytoskeletal delivery system and a cytoskeletal tethering system to stabilise the growing wall, weakened by insertion of new precursors, against osmotic stress (for discussion of the situation in present day fungi see Moore et al., 2011, pp 137-144; Read et al., 2009, 2010; Riquelme et al., 2007; Riquelme and Bartnicki-García, 2008; Steinberg, 2007; Steinberg and Schuster, 2011).
- Cell fusion primarily involves adaptation of cell wall construction functions to enable organised disassembly of two cell walls in contact (without risking osmotic stress to either cell), permitting their two cell membranes to make the two cytoplasms coextensive (see Glass et al., 2004 for discussion of present day fungi). In the ancient opisthokont cells the selective advantage of cell fusion could have been the opportunity it offers for collaboration in heterokaryons and heteroplasmons. Success could lead to the emergence of cytoplasmic (vegetative) and nuclear (sexual) compatibility/incompatibility systems (self/non-self recognition) which on the one hand would allow cytoplasmically compatible cells to exchange nuclei and form heterokaryons and on the other hand exchange of dissimilar nuclei as a prelude to sexual reproduction and all that means for evolutionary progress.
- Septum (cross wall) formation is primarily a way of protecting the cell from the hazard of loss of cytoplasmic contents following puncture of the osmotically pressurised hydrostatic system. There is, consequently, selective advantage in developing a contractile ring of actin as a way to seal damaged cells rapidly.
- After all these processes were established at the opisthokont level, this becomes a potential branch point for divergence to animals (chytrid-like to choanozoa-like), gradually losing the rigid wall and adapting the cytoskeletal organisation/vesicle trafficking originally used in wall synthesis and stabilisation to new functions of phagocytosis, locomotion and contractile cell division.
This branch event could also have been the point in time when fungi became (possibly accidentally) fixed on ergosterol as the quantitatively predominant sterol involved with controlling membrane fluidity in contrast to the cholesterol used in animals. The chytrid-like opisthokont now becomes the ‘stem-fungus’ which evolves into ancestral fungi apically-extending with the Spitzenkörper as the ultimate organising centre for hyphal extension and filamentous hyphal morphogenesis. Creating nucleated hyphae to explore and exploit the then extant biofilm and beyond the biofilm to accumulated terrestrial debris of prokaryote growth. This sequence of events (Fig. 3) allowed filamentous fungi to emerge over 1.5 billion years ago as the first crown group of eukaryotes.
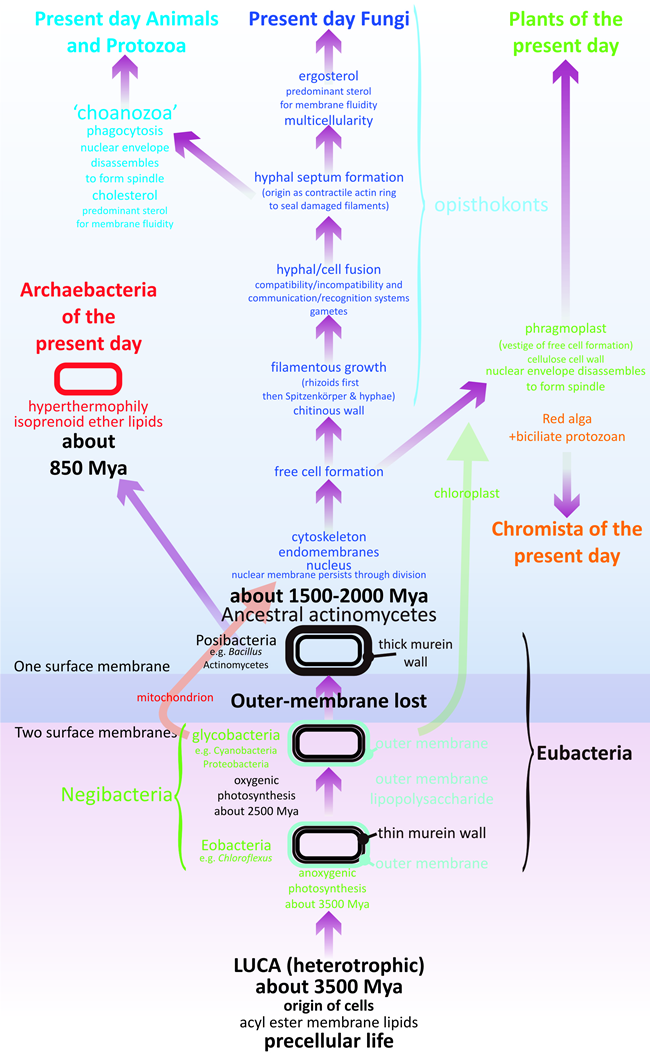 |
| Fig. 3. Emergence of a fungus-like stem for eukaryote evolution. The lower part of this diagram is based on Cavalier-Smith’s tree of life (Cavalier-Smith, 2010a; his Fig. 6), which emphasises major evolutionary changes in membrane topology and chemistry, except that the most ancient bacteria are shown here to be heterotrophic descendants of LUCA (the last universal common ancestor). Eukaryotes diverge from actinobacterial ancestors about 1500 to 2000 Mya (million years ago) and the bulk of this illustration deals with eukaryote evolution. Evolution of the most ancient stem eukaryotes are considered to depend on increasingly detailed management of the positioning and distribution of membrane-bound compartments (vacuoles, vesicles and microvesicles) by the filamentous components of the cytoskeleton (microfilaments, intermediate filaments and microtubules). Features which remain in present day organisms as characteristics of modern filamentous fungi. |
Updated December 7, 2016
