3. Rise of the fungi
Although fungal hyphae have few unique morphological features and most fungal structures are poor candidates for preservation as fossils, a respectable fossil record for fungi has been assembled in recent years. Ancient fungal fossils are found in the exquisitely-preserved Devonian Rhynie Chert of Aberdeenshire in the north of Scotland (410 million years old); easily recognisable mycorrhizal fungi from the Glomeromycota and several other fungi have been found associated with the preserved tissues of early vascular plants (Taylor et al., 1997, 2004, 2006). glomeromycotan fossils have also been found in mid-Ordovician rocks of Wisconsin (460 million years old) (Redecker et al., 2000). The age of these fossil fungi indicates that glomeromycotan fungi were present before the first vascular plants arose, when the land flora consisted of bryophytes, lichens and cyanobacteria. It is reasonable to suppose that arbuscular mycorrhizas played an important role in the success of early terrestrial plants (Blackwell, 2000; Redecker et al., 2000).
Fossil lichens have also been described from the Rhynie Chert (Taylor et al., 1997), as well as fine ascomycete specimens showing ascospore development and perithecia (Taylor et al., 2004), from which we can infer a fully evolved fungal developmental biology able to produce extreme hyphal differentiation, cell signalling, cell sorting, pattern formation and formation of tissues with different functions, all of which are typical of the present day. Differentiated conidiophores emerging through a plant epidermis are also shown; suggesting a fully adapted plant pathogen at an extremely early stage in plant evolution. Chytrids are “…probably the most common microbial element…” in the Rhynie Chert (Taylor et al., 2006) and include fully differentiated chytrid zoosporangia, which suggest the inference that at the time the sediments were laid down the complete cell biology of the chytrids (thallus, rhizoids, free cell formation, motile zoospores, etc.) was fully established. Also among the Rhynie Chert specimens are examples of chytrids parasitising other fungi, showing, again, that 410 million years ago the fungal life style was so firmly established that fungi were parasitising other fungi. The fungus-like lifestyles represented in the Rhynie Chert specimens include some like the Oomycota of the present day. Several of these specimens show an antheridium contacting an oogonium, clearly demonstrating the operation of a fully differentiated sexual reproduction process including differentiated male and female gametes and all that goes with them, including sexual hormones, hormone receptors and male to female cell targeting.
By far the most impressive fungi of the Ordovician/Devonian period are specimens of the fossil genus Prototaxites, which were terrestrial organisms found from the mid-Ordovician (460 million years ago) to the early Devonian, suggesting that they lasted a period of at least 40 million years (Hueber, 2001; Boyce et al., 2007). These fossils are among the ‘nematophyte phytodebris’ that constitutes the earliest evidence for terrestrial organisms. This nomenclature was assigned in the middle of the 19th century and has no relevance to present day understanding of taxonomy; though it does indicate that confusion over the identification of the material is over 150 years old (see discussion in Hueber, 2001 and Taylor et al., 2010). Prototaxites specimens are generally large; over a metre wide (Wellman and Gray, 2000) and up to 8 m tall (Hueber, 2001). Some of the earliest examples were tree-like trunks constructed of interwoven tubes <50 µm in diameter (concentrically arranged in transverse sections), and were interpreted to be small coniferous trees, though we now know that environments at the time Prototaxites was fossilised did not (yet) include large vascular plants.
Prototaxites was also so common that it was a major component, both in terms of abundance and diversity, of its habitat andwas by far the largest organism then existent. These early terrestrial ecosystems were still dependent on the more ancient primary producers, cyanobacteria, eukaryotic algae, lichens and mosses, liverworts, and bryophytes. Isotope ratio mass spectrometry of individual Prototaxites fossils provides the most compelling evidence that Prototaxites was a fungus (Boyce et al., 2007; Hobbie and Boyce, 2010); I personally consider this evidence conclusive. These analyses show that carbon isotope ratios (12C:13C) of individual Prototaxites fossils varied too much for them to be photosynthetic primary producers of any sort (Boyce et al., 2007), indicating that Prototaxites was a heterotroph (saprotroph) that digested isotopically heterogeneous substrates; it was a consumer and recycler. Hobbie and Boyce (2010) demonstrated a similar large range of carbon isotope values among fungi, particularly saprotrophic fungi, of a present day environment resembling the Early Silurian and Devonian landscapes where Prototaxites occurred. Hueber’s (2001) critical examination of the microscopic anatomy of Prototaxites found similarities with the trimitic system of hyphae evident in present day basidiomycetes; he states: “Prototaxites is nomenclaturally valid... This report has a triple purpose: (1) to name, as neotype, a recognizable specimen [of Prototaxites] collected by Dawson for which the locality and stratigraphic data are known, (2) to redescribe the genus as structurally composed of three interactive forms of hyphae, i.e. large thin-walled, septate, branching, generative hyphae; large thick-walled, non-septate, skeletal hyphae; and small thin-walled, septate, branching, binding hyphae, which combine to form a gigantic, phototropic [more likely gravitropic in my view], amphigenous [= a hymenial hyphal layer of present day Ascomycota and Basidiomycota that extends over the entire surface of the spore-producing body], perennial sporophore with saprobic nutrition, and (3) to classify it in the Kingdom Fungi.” (Hueber, 2001; abstract, with comments from me in square brackets).
Taking all this evidence together the conclusion is inescapable to me that these enormous fossils, which were the largest land organisms to have lived up to their point in time, were actually giant terrestrial saprotrophic fungi, with affinities (dolipore septa, clamp connections and sterigmata) to present day Basidiomycota. Consequently, I believe that convincing fossil evidence shows that fungi were important, even dominant, members of terrestrial ecosystems approximately 500 million years ago. Well-developed filamentous fungi must have first appeared a long time before that, however. How long would it take the ancestors of the Rhynie Chert water moulds, chytrids, Glomeromycota, lichens and Ascomycota to evolve the capability to form structures microscopically indistinguishable from those of the present day? How long would it take the ancestors of Prototaxites to evolve an 8 m tall club-fungus? Guessing could push ‘well-developed filamentous fungi’ back in time to about 700-800 million years ago. But there are older fossils than that, even though they may be disputed.
Butterfield (2005) assigned fossils extracted from formations in northwestern Canada, the deposition of which has been dated to between 800 and 900 million years ago, to the form-genus Tappania; describing the organism as: “…an actively growing, benthic, multicellular organism capable of substantial differentiation. Most notably, its septate, branching, filamentous processes were capable of secondary fusion, a synapomorphy of [trait shared by] the ‘higher fungi’ [of today]. Combined with phylogenetic, taphonomic and functional morphologic evidence, such ‘hyphal fusion’ identifies Tappania reliably, if not conclusively, as a fungus, probably a sister group to the ‘higher fungi’ [Dikarya], but more derived than the zygomycetes.” (Butterfield, 2005; abstract).
The form genus fossil Tappania is widespread, having been found in ancient shoreline carbonaceous shale deposits in Australia, Canada, and China. Specimens fossilised nearly 1.5 billion years ago in shales in northern Australia have been described (Javaux et al., 2001). Javaux et al. (2001) go no further than to state that the systematic relationships of Tappania are uncertain, but its distinctive morphology indicates that: “…the cytoskeletal architecture and regulatory networks that characterize living [eukaryote] protists…” were in place in organisms fossilised 1.5 billion years ago. However, Butterfield (2005) discusses these and other putative pre-Devonian fungi and concludes that “…there is a case to be made for an extended and relatively diverse record of Proterozoic fungi.” Cavalier-Smith (2006; pp. 983-984) agrees with Butterfield’s (2005) identification of Tappania as sporangial entities broken from a branching trophic hyphal network, but does not agree that these fossils are probably fungi. He suggests they could instead be actinobacterial pseudosporangia; I do not find this convincing.
The large spheroidal microfossils shown in these Tappania papers are usually described as ‘vesicles’. Butterfield’s (2005) specimens, after being dissolved into slurry with 30% HF and filtered through a 62 μm mesh sieve, are described as follows: “… from 30 μm … to over 400 μm … in transverse dimension … Processes are typically heteromorphic and range from 0.3 μm … to >4 μm … in diameter. In some instances, simple cylindrical processes may be distributed relatively uniformly over the vesicle surface …; in others, they occur as isolated knoblike buds … or elongate filamentous extensions …. In most cases, however, the processes are further distinguished by distal branching … and a capacity to form closed loops through secondary fusion. This fusion appears to be relatively indiscriminate and gives rise to a wide range of expression: occasionally the processes return directly to the vesicle to form simple loops …; in other cases they have fused either with themselves … or, more commonly, with other processes …, resulting in a distally interconnected network... Multiple layers of process networks are also developed, sometimes to the extent of obscuring the central vesicle …” (Butterfield, 2005; p. 167) (see Fig. 2).
This is quoted in detail because I have spent most of my research life cultivating a basidiomycete fungus (Coprinopsis cinerea) which, in common with many other present day ascomycete and basidiomycete soil fungi produces abundant sclerotia in and on mycelial cultures. We have described these: “…Mature aerial sclerotia were dark brown to black, more or less spherical and variable in size although most were in the range 100-250 μm in diameter … three tissue layers were apparent - the outer diffuse layer, the rind and the medulla. The outermost diffuse layer … was composed of apparently dead hyphal cells whose cytoplasm was reduced to membrane fragments and vesiculate structures. Many had crenulate cell walls which may indicate they were damaged during preparation for sectioning. This outer layer, though only loosely attached and often sloughed off during fixation, was always present in mature aerial sclerotia and is therefore regarded as an integral part of their structure.” (Waters et al., 1975a; p. 201; see also Waters et al., 1975b) (see Fig. 2).
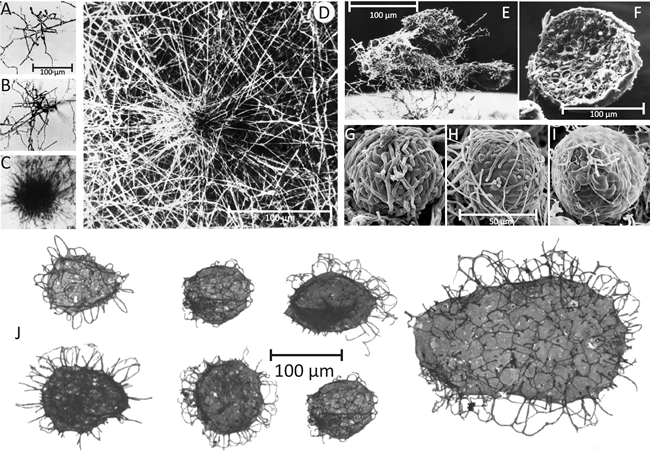 |
| Fig. 2. Comparison of present day fungal sclerotia (top panel) with Tappania sp. fossils (bottom panel). A-D, Culture slide (microcosm) preparations of developing sclerotia of Coprinopsis cinerea. A: the earliest recognisable stage showing the centre of sclerotium initiation. B: increased size of structure as branching proceeds to form a very immature sclerotium. C: immature sclerotium showing main body with hyphae of determinate growth and the ‘long hyphae’ that appear to radiate outwards but are actually targeting inwards towards the developing sclerotium. D: Scanning electron micrograph of immature sclerotium at a stage similar to that shown in C, which shows more clearly that the long hyphae are part of the long distance nutrient translocation network that supports development of the sclerotium (A-C are light micrographs; all images from Waters et al., (1975a and b) by permission of John Wiley and Sons). E: Scanning electron micrograph of a group of three aerial sclerotia of C. cinerea in side view, relatively undisturbed and still showing the investing layer of mycelial hyphae and obvious aerial habit. Photographed by Dr H. Waters. The mycelial hyphae that surround the developing sclerotium, which seem so substantial in D and E, are easily lost during preparation for microscopy. F: Scanning electron micrograph of a freeze-fractured aerial sclerotium of C. cinerea. This specimen is one of a collection of sclerotia that were scraped from the surface of a Petri dish culture, suspended in glutaraldehyde fixative, filtered through cotton, dehydrated into acetone, then ground in a mortar and pestle under liquid nitrogen (to fracture the sclerotia and reveal their internal structure); not much of the investing layer of aerial mycelium survives this treatment. Although the chemistry is very different, the physical processes involved in this preparation are quite similar to those recorded for the preparation of Tappania fossil specimens (see text). This specimen photographed by Dr F.V. Hereward. G to I: scanning electron micrographs of the (smaller) sclerotia of Byssocorticium coprophilum (MycoBank reference Mb449580), which show more clearly that sclerotia are a ‘ball’ of filamentous hyphae and that constituent cells (compartments) of those hyphae differentiate as the sclerotium matures. Images reprinted by permission from Dr J.A. Stalpers; they originally appeared on the www.mycobank.org website. J: Tappania sp. fossils from the Wynniatt Formation on Victoria Island, northwestern Canada. Specimens are generally described as ‘vesicles’ with ‘processes’; some are described as ‘now flattened’. These specimens are between 800 and 900 million years old. Images (here adjusted to the same scale) from Butterfield (2005). Note the Tappania fossil ‘vesicles’ have a surface sculpturing beneath the ‘processes’ similar to that evident in present day fungal sclerotia beneath the investing hyphal layer (particularly evident in images G to I). |
Although I’ve never seen them after a billion years of preservation followed by dissolution into hydrofluoric acid, I have handled a great many fungal sclerotia in various states: fresh, in actively growing cultures including microcosms, desiccated in old stored cultures with collapsed and twisted outer-layer hyphae, fixed for LM and TEM, and critical-point dried for SEM. This first-hand experience convinces me that the Tappania ‘vesicles’ illustrated by Javaux et al. (2001) and Butterfield (2005) could have been the sclerotia of filamentous saprotrophic moulds and soil fungi.
If true, this interpretation implies that filamentous moulds with affinities to the Ascomycota and/or Basidiomycota of the present day and able to regulate hyphal branching and hyphal interactions with sufficient finesse to assemble multicellular survival and, perhaps, reproductive structures, were common and widespread up to 1.5 billion years ago.
Updated December 7, 2016
