8. Meet the Fungiflexes
The indications are, therefore that these ‘fungal growth co-ordinating factors’ are specifically-modified intermediary metabolites, and in particular, modified sugar molecules.
That they are no more exotic than this would fit in well with the claim that septum formation in the most primitive fungal hypha was the process that invented eukaryotic multicellularity (Moore, 2013a).
Moore (2013a) argued that multicellular fungal hyphae were the first successful multicellular eukaryotic tissue structures, appearing about 1.5 billion years ago. The development of the septum across the filament has the immediate selective advantage of protecting the filament against the disaster of wall damage opening the cell to the environment. But it also simultaneously invents compartment (‘cell’) differentiation because the filament extends by growth at its apex. So directly a septum is formed, the ‘front’ compartment is the growing apex and the sub-terminal compartment is no longer part of the growing apex. It is, specifically, sub-terminal; and may be capable of intercalary wall growth, but is not engaged in apical growth. Moore claims that it was the fungal life style that began the ancestral eukaryote’s tendency towards multicellularity, possibly at a time even before kingdom Fungi was fully delineated. Those early eukaryotes first worked out how to make and use multicellular filaments (now called hyphae), then evolved the mechanisms to control those key features of multicellularity (now known as developmental biology or morphogenesis). The key features being differentiation of the compartments (‘cell differentiation’), patterns of distribution in space and time, and establishment of ‘body plans’ that feature in the development of multicellular structures like fungal fruit bodies; and which will feature later in geological time in plants and, eventually, animals too (Moore, 2013a)[view Publisher's page] [view Amazon page]. |
If the simplicity of form and function suggested by distant evolutionary events is even partially accurate, then the chemicals most readily available for recruitment as growth control agents in the primitive metabolism of the time were probably the intermediary metabolites themselves.
Metabolism was already focussed on hexose sugars and featured sugar phosphates, amino sugars, sugar alcohols and sugar acids, and, of course, synthesis of chitin for the fungal walls made use of repeating units of N-acetylglucosamine. Long before the need to create signalling molecules arose, the enzymes and pathways existed to form sugar derivatives that could eventually serve as specific regulators of the enzyme systems involved in wall structure and/or architecture so as to control the shape and form of hyphae and the morphogenesis of the tissues the hyphae comprised.
Distilling all these hints and suggestions brings us to the conclusion that the Fungiflex activities we extract so readily by infusing intact tissues in water at room temperature, and detect so readily in our ‘stipe bending’ bioassay are:
- a family of molecules, certainly two, possibly more;
- most probably 6-deoxy hexoses (and therefore also interpretable as methyl-pentoses);
- probably substituted with amino and/or amido groups;
- probably N-acetylated;
- possibly phosphorylated, and maybe sulfated.
The NMR spectra of Fungiflex extracts are strongly characteristic of a laevorotatory 6-deoxy hexose sugar, probably L-fucose, possibly L-rhamnose. With the evidence for substituents of the molecules shown earlier (see section 5), we can illustrate our speculations about likely Fungiflex molecular structures in the following panel.
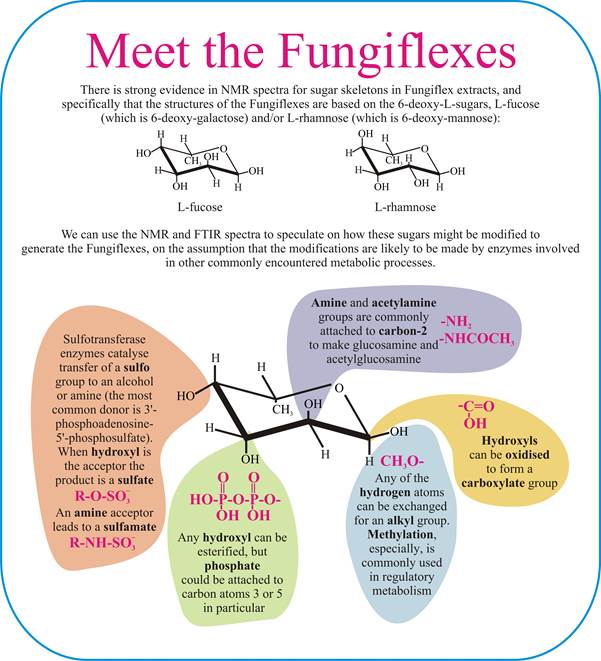
The sugar's anomeric signals are weak, but the FTIR spectrum of Fungiflex shows evidence for a carboxylate ion. Monosaccharides with a carboxyl group are known as sugar acids, and are the result of oxidation of one or more hydroxyl groups. An interesting example, and perhaps relevant in light of the weak anomeric signals detected, is galactonic acid in which the carboxylate group is at C1 of galactose.
Other aspects of the FTIR spectra could provide evidence for the presence of a diphosphate group and the elemental analyses are not inconsistent with the presence of sulfur.
| Our thanks are due to Dr Rebecca J. Moore for interpretation of FTIR spectra and Dr Bryan Eastwood for interpretation of NMR spectra. However, responsibility for the speculations about Fungiflex structures implicit in this figure above rests with the authors. |
Copyright © David Moore & Lily Novak-Frazer 2016
