6. Some reminders about the structures of sugars
Without giving too much of the plot away, it's clear that sugar molecules are critically important aspects to our story. Some background information on the following page will give you an in-depth understanding of the terminology used in sugar chemistry, and place our interpretations in context.
However, if you want to skip this section (and maybe return to it later if you need), you could ‘cut to the chase’ without further ado, and look at the page entitled ‘Meet the Fungiflexes’.
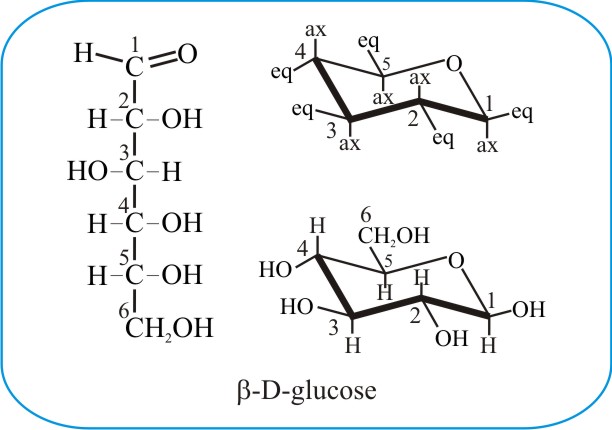
The images above depict the glucose molecule. On the left is the linear structure, or Fischer projection, of D-glucose, while on the right is the C1-chair form of the same molecule, demonstrating the five-carbon pyranose ring. The five carbon atoms of the pyranose sugar (as indicated by the numerals) are connected into a six-atom heterocyclic ring through formation of a stable hemiacetal or hemiketal (called a lactol). This occurs when a spontaneous intramolecular reaction takes place between the OH group of carbon 5 (C5) and the oxygen of the carbonyl group (>C=O) of carbon 1 (C1) in this example. The ring is planar (that is, it’s fairly flat) and the figure above tries to depict this in three-dimensions by showing the bonds closest to you, the viewer, emphasised with thickened lines. In an aqueous solution of glucose about 97% of the glucose molecules are in this ring form.
A few important structural features of sugar molecules need to be defined. Chiral molecules are those whose mirror images cannot be superimposed. They contain at least one carbon atom with four non-identical substituents. In the Fischer projection above, there are four (4) chiral carbons (C2-C5) and one non-chiral carbon (C6). C1, the carbonyl carbon, is called the anomeric centre as it reacts with C5 to form the cyclic form of glucose.
In the cyclic glucopyranose molecule depicted above, C1, which is now a chiral centre, has four different substituents in this form: the adjacent carbon 2 (C2), the oxygen that’s part of the ring, a proton [H] and the hydroxyl [OH]). More importantly, it is also an anomeric centre because in this cyclised form, the molecule can take up two different geometries or anomers, named the alpha and beta forms.
Alpha and beta glucopyranose molecules differ only in the direction that -H and -OH groups point on the anomeric carbon (or C1) when in the ring or cyclic form. Alpha glucopyranose has its C1-OH (hydroxyl) group on the axial bond (i.e. pointing downwards, away from the plane of the ring), whereas the C1-OH of beta glucopyranose is attached through the equatorial bond and is held slightly above the ring. The axial (labelled ax) and equatorial (eq) bonds are shown in the skeletal structure at top right of the above panel.
The anomeric carbon is particularly important as it is the carbon involved in linking sugar monomers together to form polysaccharides.
Considering the Fischer projection of glucose shown above, a D or L designation is denoted by the configuration at the chiral centre furthest from the anomeric centre.
In glucose, the aldehyde group is C1, also the anomeric centre, and the most distant chiral carbon is C5. The rule is ‘if the chiral centre furthest from the anomeric centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar’. The open chain molecular representations of D & L sugars are mirror images of one another.
| The sources of the D- and L- labels were the Latin words dexter (on the right) and laevus (on the left) as when this scheme was devised (in the early years of the twentieth century by the German organic chemist Emil Fischer) the form of the molecule (glyceraldehyde being the object of study) that rotated plain polarized light clockwise (also designated ‘+’) was arbitrarily labelled ‘D’ (for dextrorotatory). The other form of the molecule was found to rotate polarized light anticlockwise (also designated '-') and was called ‘L’ for laevorotatory. |
In the ring-form representation in the panel at the top of this page, the D-sugar has carbon 6 (C6) directed above the ring, while the L-sugar has that carbon directed below the ring. Generally, the highest numbered chiral carbon (typically to the left of the oxygen in chair or Haworth projections) determines whether or not the structure has a D- or L-configuration.
D-glucose is depicted in the illustration above, and is the most common form of the molecule in nature (and this is true for most other naturally occurring sugars). In L-glucose (depicted immediately below) the hydroxymethyl group (- OCH3) and the proton (-H) are interchanged on C5. L-Glucose is rarely found in nature, but can be chemically-synthesized and its taste is indistinguishable from D-glucose. However, L-glucose cannot be metabolised by living organisms as a source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in glycolytic metabolism.
In the following panel we show the molecular structures of a selection of commonly-encountered sugars and some chemically-modified sugars. Our motive here is to illustrate just a little of the enormous diversity represented among the hexose sugars. We also want to illustrate how tiny differences in the geometry of the molecules are sufficient to distinguish between completely different sugars. Can you spot the difference(s) between glucose, mannose and galactose in the left-hand column, below?
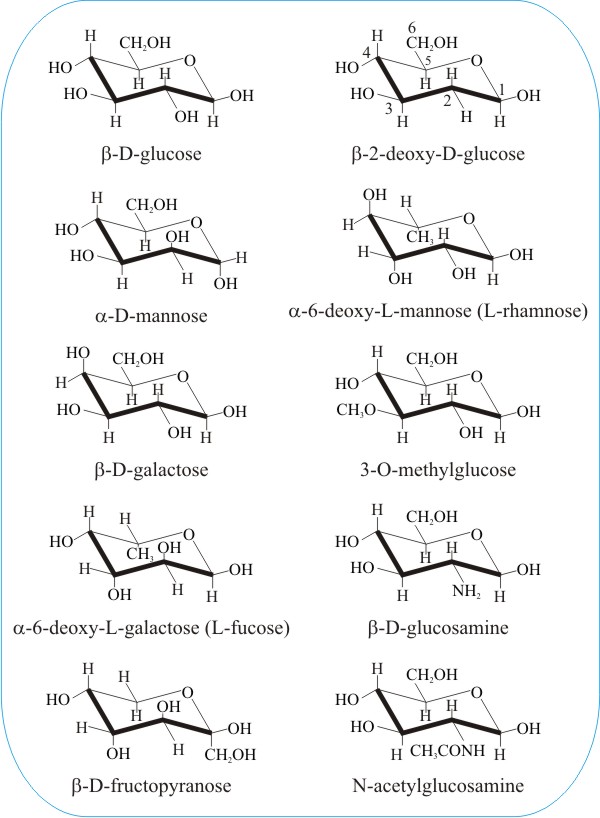
And then there's fructose. Shown at the bottom of the left-hand column in the above panel; compare it with the glucose molecule at the top of that column. Doesn't look much like glucose, does it? And yet fructose is also called fruit-sugar. It's paired with glucose to make the common plant disaccharide sucrose (so every sucrose molecule you digest produces a molecule of fructose). But fructose is sweeter than either glucose or sucrose and high-fructose corn syrup (HFCS) industrially produced from sucrose is widely used in soft drinks and other 'sweet treats'. Its consumption has risen in parallel with the epidemic of obesity, which suggests a relation, and implicates dietary fructose as a potential risk factor for diabetes and cardiovascular disease.
| If you want more information about the dangers of fructose in the human diet, you could start with this (free) PDF download from the website of the Journal of the American Society for Clinical Nutrition (the URL is http://ajcn.nutrition.org/content/86/4/895.full.pdf+html) CLICK HERE to open this file in a new window. |
You might well wonder how a sugar structure that looks so different from that of glucose can be a dietary concern. The answer is that fructose is an essential component of the glycolytic metabolism of glucose. Fructose 6-phosphate is produced by isomerisation of glucose 6-phosphate, which is in turn further phosphorylated to fructose-1,6-bisphosphate. So although there is no biological need for dietary fructose, it will be metabolised as a source of carbon and energy. Fructose is metabolized by phosphorylation on the 1-position and how this might be accomplished by normal glycolytic enzymes is indicated in the next panel.
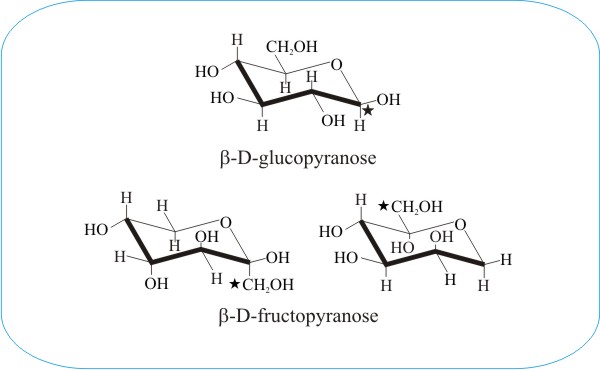
The above comparison of the pyranose ring forms of β-D-glucose and β-D-fructose demonstrates the important concept of the conformational flexibility of monomeric sugars. The fructopyranose ring is shown on the left in the conventional orientation and, in the right-hand diagram, the molecular structure has been inverted and rotated to present a view which provides a closer comparison with glucose. In each diagram the C1-carbon atom is indicated with a star («). The structural similarity with glucose is evidently now sufficient for enzymes that normally add phosphate groups to carbon-6 of glucose to add phosphate groups to carbon-1 of fructose.
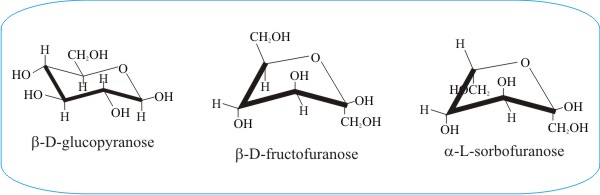
Another example of conformational flexibility is shown in the panel immediately above, which compares the pyranose ring form of glucose with the furanose ring forms of fructose and sorbose. The furanose structure is a five-membered heterocyclic ring, consisting of four carbon atoms and one oxygen atom. The conventional diagram shows the anomeric carbon to the right of the oxygen.
The furanose sugar will be either an α or β anomer, depending on which direction the anomeric hydroxyl group is pointing. In a D-configuration furanose, α configuration has the hydroxyl pointing down, and β has the hydroxyl pointing up. It is the opposite in an L-configuration furanose. In aqueous solution there will be an equilibrium mixture of α and β configurations due to mutarotation at the anomeric carbon.
Copyright © David Moore & Lily Novak-Frazer 2016
