6.6 The glycoprotein component
Within the wall, the branched glucans are cross-linked together and to chitin; in addition, the glucan has mannoproteins attached to it, and this introduces the idea that the protein components of fungal walls are of considerable importance, too (De Groot, Ram & Klis, 2005; Klis, Boorsma & De Groot, 2006). In the yeast cell wall (S. cerevisiae and C. albicans) proteins account for 30-50% of the dry weight; the N. crassa hyphal cell wall comprises approximately 15% protein by dry weight and generally in filamentous fungi cell walls contain 20-30% protein.
Most cell wall proteins are glycoproteins that are modified with N-linked and O-linked oligosaccharides. These are proteins that have an amino-terminal signal peptide in their primary sequence which attaches the translating ribosome to the endoplasmic reticulum (ER) membrane. As the polypeptide is translated, the protein is extruded into the ER lumen; and this is the start of their journey through the secretory pathway (Fig. 3.)(discussed in the ‘mRNA translation and protein sorting’ section in Chapter 5, CLICK HERE to view the page). During translocation of the protein through the secretory pathway glycosylation occurs by covalent linkage of large, branched oligosaccharide structures to what then becomes a glycoprotein.
N-linked oligosaccharides are added to asparagines located in peptides carrying the sequence motifs: asparagine-any amino acid-serine and asparagine-any amino acid-threonine. The branched oligosaccharide contains N-acetylglucosamine, mannose and glucose, and is transferred to the protein ready made from a dolichol lipid donor (dolichol is a general name for a polymer consisting of various numbers of isoprene units; see Section 10.13 CLICK HERE to view the page). This oligosaccharide structure is synthesised on the ER membrane by sequential addition of sugar residues by glycosyltransferase enzymes to the dolichol lipid group which tethers the growing oligosaccharide chain to the membrane. When completed, the branched oligosaccharide is transferred to the nascent protein (during translation) by the N-oligosaccharyltransferase complex catalysing formation of a glycosidic bond between the first N-acetylglucosamine in the oligosaccharide chain and the amino group of the target asparagine.
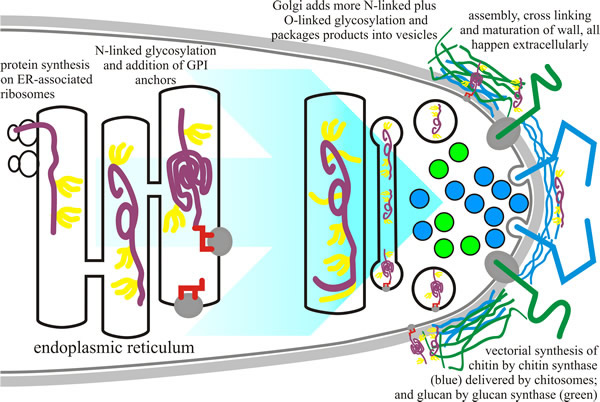 |
| Fig. 3. Biosynthesis of cell wall components. Glycoprotein synthesis begins in the endoplasmic reticulum (ER; protein shown in purple) with the addition of N-linked oligosaccharides (yellow) during translation. GPI anchors (red) are also added to some proteins in the ER. In the Golgi apparatus, the glycosyltransferases modify the proteins further by addition of sugars to generate O-linked oligosaccharides and to extend N-linked oligosaccharides. Glycoproteins are secreted into the cell wall space where they are integrated into the cell wall structure. The chitin (blue) and glucan (green) components of the cell wall are vectorially synthesised on the plasma membrane and extruded into the cell wall space during their synthesis. The various components of the wall are cross linked together in the cell wall space by cell-wall-associated glycosylhydrolases and glycosyltransferases (based on a figure in Bowman & Free, 2006). |
Once attached to the protein, the oligosaccharide is extensively modified during transit of the glycoprotein through the ER and Golgi (see section on The endomembrane systems in Chapter 5, CLICK HERE to view the page). Modifications include both removal and addition of sugars, and account for the great diversity in N-linked oligosaccharides of glycoproteins. N-glycans are the major form of mannoprotein modification in the yeasts Saccharomyces cerevisiae and Candida albicans and consist of a core structure, which is similar in all eukaryotes and is further elaborated in the Golgi to form an outer chain comprising a linear α1,6-mannan backbone that is highly branched with α1,2- and α1,3-containing side chains In yeasts (S. cerevisiae and C. albicans), addition of long chains of α1,6-linked mannoses with short branches of α1,2 and α1,3 mannoses to the N-linked oligosaccharides generate the mannoproteins characteristic of yeast cell walls (Fig. 2C, above). In N. crassa and A. fumigatus, the N-linked oligosaccharides are modified to become galactomannans: being processed by Golgi resident enzymes into an α1,6-linked mannose core with α1,2-linked mannose side chains terminated with a variable number of β1,5-linked galactose residues (Bowman & Free, 2006; Lesage & Bussey, 2006).
O-linked mannans of fungi tend to be short, linear chains composed of α-linked mannose sugars. The synthesis of other decorating polymers is less well understood, but they include α1,3-glucans, β1,6-glucans and a lengthening list of heteropolymeric polysaccharides (that is, polysaccharides composed of one, two, or several different monosaccharides, in straight or branched chains). Most of what we know about O-linked glycosylation comes from work on Saccharomyces cerevisiae, where synthesis begins in the ER with the addition of a single mannose to the ‘hydroxy’ oxygen of selected serine and/or threonine residues in the polypeptide which is being translated. This sugar is transferred from dolichol-mannose by a family of protein-O-mannosyltransferase enzymes. The remainder of the O-linked oligosaccharide elongation is done by mannosyltransferase enzymes in the Golgi apparatus. In Neurospora crassa, the O-linked structure contains galactose as well. O-linked oligosaccharides of fungi are not branched, and they are not as long as N-linked glycans.
A special form of glycosylation is the GPI anchor; in this case glycosylation serves to attach a hydrophobic lipid anchor to proteins that carry a particular carboxy-terminal signal sequence. The GPI anchor is a lipid and oligosaccharide-containing structure that directs and localises the protein to the plasma membrane. The attachment is done by a protein complex located in the ER membrane, known as GPI transamidase (though the complex consists of about twenty different proteins), which recognises the carboxy-terminal signal sequence as the translated polypeptide migrates through the ER membrane, and transfers the pre-assembled GPI anchor onto the newly generated carboxy-terminus of the protein.
Genetic analyses have shown that many of the genes in the GPI anchor biosynthetic pathway are essential for viability in both yeasts and filamentous fungi, suggesting that the GPI-anchored proteins are required for integrity of the cell wall. Yeast genomes can contain from 28 to 169 GPI-anchored protein sequences (most in the pathogenic Candida albicans), with 74 and 87 genes in the genomes of Aspergillus nidulans and Neurospora crassa, respectively. Known GPI-anchored proteins include glycosylhydrolases, glycosyltransferases, and peptidases, all of which are likely to contribute to cell wall synthesis and remodelling. Clearly, GPI-anchored proteins are critical for the formation and maintenance of the cell wall (Bowman & Free, 2006; Lesage & Bussey, 2006). (GPI anchors and their association with the fungal wall is mentioned in Chapter 5, CLICK HERE to view the page).
Most cell wall proteins are structurally integrated into the wall, being covalently linked to chitin and/or glucan through sugars in their N- and O-linked oligosaccharides and/or in their GPI anchor. The proteins recognised as ‘proteins with internal repeats’, or (PIR)-proteins crosslink β1,3-glucan chains through ester linkages, forming the protein-polysaccharide complex β1,3-glucan–PIR- β1,3-glucan and are found throughout the inner layer of the wall (Yin et al., 2007). Proteins in the cell wall contribute to maintenance of cell shape, adhesion, protection against environmental stress, absorption of molecules, transmission of intracellular signals, and receipt of external stimuli, as well as the synthesis and remodelling of wall components (Adams, 2004; De Groot et al., 2005; Klis et al., 2006).
A family of aspartic peptidases (called yapsins) are important for cell-wall assembly and/or remodelling. They are tethered to the membrane and the wall by a GPI anchor and are found only in fungi. No natural substrates have yet been identified for any of the yapsin enzymes, but they are thought to function in two ways: activation of periplasmic enzymes by endoproteolysis to involve them in glucan synthesis (perhaps in response to changing environmental conditions), and/or to release GPI-anchored enzymes from the wall when active cell wall assembly is no longer required by shedding the proteins by endoproteolysis. Some yapsins of Candida spp. have been implicated in virulence and infection by influencing adherence to epithelial cells (Gagnon-Arsenault et al., 2006).
Another interesting group of cell wall-associated proteins are cytoplasmic, even mitochondrial, enzymes, which do not have a signal sequence for export across the cell membrane. In most natural situations, the outer hyphal wall has a layer of such proteins, which have been called moonlighting proteins (Jeffery, 1999). These have been assumed to be contaminants of fungal cell wall preparations in the past, because finding enzymes of glycolysis or other metabolic pathways hanging around in the wall is, to say the least, unexpected.
Another possibility is that because fungal cell walls have a significant capacity to absorb soluble proteins from the environment, fungal surfaces may be contaminated with cytoplasmic proteins picked up from the environment. However, there is increasing evidence that they really are resident proteins cross-linked into the cell wall. Obviously, such proteins could provide the ‘wall organelle’ with range of functions but not enough is known yet to form a coherent picture. It is abundantly clear, though, that moonlighting proteins occur very widely in many different organisms. The first examples of moonlighting proteins were described in the late 1980s, when it was found that the crystallin structural proteins in the lens of the vertebrate eye were enzymes of intermediary metabolism. For example, duck ε-crystallin is lactate dehydrogenase, and turtle τ-crystallin is the glycolytic enzyme α-enolase. Any metabolic function for such enzymes in the lens of the eye is highly unlikely. Instead they probably serve a structural function in the lens where they accumulate to very high concentrations (Huberts & van der Klei, 2010). Moonlighting proteins are multifunctional proteins that perform several, often unrelated, and completely independent functions. This is why they are given that name, by analogy to moonlighting people who have multiple jobs.
Updated July, 2019
