7. Sugars as regulatory molecules
The data in Section 5 (Chemistry of the Fungiflexes) demonstrate that these ‘fungal growth co-ordinating factors’ that we have called 'Fungiflexes' are specifically-modified sugars. That sugars should play multidisciplinary roles in fungal development and extracellular communication, as well as being metabolic intermediates is significant and may not be too surprising.
More surprising to the senior author (David Moore, that is, and in this context the word ‘senior’ should be taken to mean ‘more ancient’) is that the findings of this Fungiflex research, which was done close to the end of his career, echoed resoundingly research on carbohydrate metabolism in Coprinopsis cinerea that was done at the start of his research career some 30 years earlier.
David Moore has reviewed his research career in the book ‘Coprinopsis: an Autobiography’ (Moore, 2013b)[view Amazon page], but he has described his researches in these distant days for us and we present some extracts of this here.
On this page we summarise David Moore's own account of his research on fungal carbohydrate metabolism in the 1960s and 1970s. If you want to read the complete original CLICK HERE to open that page in a new window. Note that on both pages, if you click on the hyperlink citations in the text (which are shown as underlined blue text) you can download the publication cited as a free PDF into a new window. CLICK HERE for a PDF which is a more general source of information about the effects of unusual sugars on fungi. |
David writes: 'When I took up my first job as Assistant Lecturer in Genetics in the University of Manchester, I initiated a study of sugar metabolism. I first determined the spectrum of carbon and energy sources that could be used by Coprinopsis cinerea (named Coprinus lagopus at the time)(Moore, 1969) and then examined the effects of a number of sugar analogues on growth of the organism.'
The then-existing literature suggested that sugar analogues usually cause inhibitions to the growth of fungi by being used in metabolism in place of glucose. For example, hexokinase phosphorylates the glucose analogue 2-deoxy-D-glucose (deGlc), and its 6-phosphate inhibits the activity of other glycolytic enzymes, especially phosphohexose isomerase and glucose 6-phosphate dehydrogenase. Formation of deGlc-6P is the usual limit of metabolism of this sugar analogue; accumulation of the phosphate ester leads to a considerable drain on phosphate pools. ATP levels decline drastically and deGlc rapidly initiates degradation of purine nucleotides. In most fungi, polysaccharide synthesis is affected; preformed wall material is eroded while synthesis of new wall components is prevented.
Another sugar analogue, L-sorbose is not phosphorylated, and the biochemical basis of inhibition is more obscure. Some enzymes involved in polysaccharide synthesis are sensitive to inhibition by sorbose. But this abnormal sugar seems to affect the controls of wall synthesis because a characteristic of growth on this sugar is formation of an abnormally thick cell wall. This seems to lead to the mycelium exhibiting an abnormal growth form in which cells are much shortened and branching frequency is increased. As a result, sorbose and related compounds are called paramorphogens.
David continued: 'I found that 2-deoxy-D-glucose seriously inhibited the growth of Coprinopsis cinerea, resulting in some correlated morphological changes and suggesting the involvement of the sugar in cell wall metabolism.'
For example, under the influence of 2-deoxy-D-glucose (deGlc), outgrowths destined to become clamp connections in Coprinopsis dikaryons grew away from parent hyphae and became established as monokaryotic branches (Moore & Stewart, 1971a).
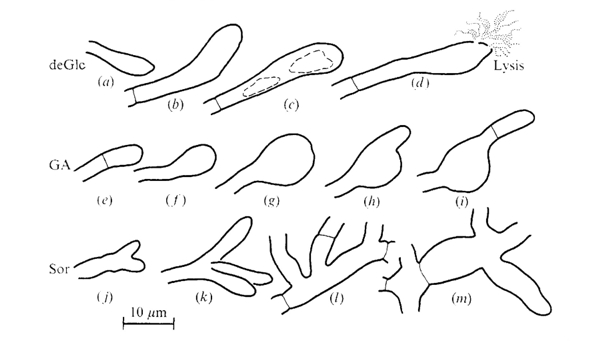
The morphologies of Coprinopsis cinerea hyphae grown on media containing inhibitory concentrations of the hexose analogues 2-deoxy-D-glucose (deGlc), D-glucosamine (GA), and L-sorbose (Sor) were illustrated by Moore & Stewart (1972) as shown in the figure below. In summary: the drastic effects of deGlc, GA and Sor shown in this figure illustrate clearly the morphogenetic changes in hyphal growth, leading the authors to conclude that 'processes which control the shape of the [hyphal] compartment must be very closely related to the processes which determine the chemical composition of the hyphal wall.’
|
Shapes assumed by terminal and intercalary compartments of hyphae of Coprinopsis cinerea grown on media containing inhibitory concentrations of hexose analogues. Drawings are tracings from photographs of individual hyphal compartments observed with oil immersion microscope objectives. The figures are arranged in a likely developmental series but individual hyphae were not followed through the development of these aberrations. Note the vacuolation and eventual lysis of swollen terminal cells which occurred on deGlc; the extreme swelling of terminal compartment on GA; and the frequent branching and giant mycelial cells seen on Sor. The following media were used: (a), (b), (d), 5 mM Glucose + 20 mM-deGlc; (c), 5 mM-Acetate + 0.1 mM-deGlc; (e), 5 mM Glucose + 15 mM GA; (f), (h), 5 mM Fructose + 0.2 mM GA; (g), (i), 40 mM Glucose + 80 mM GA; (j), (m), 5 mM Acetate + 2 mM Sor: (k), (I), 5 mM Glucose + 75 mM Sor. Figure and legend from Moore & Stewart, 1972. |
Having shown that sugar analogues inhibited Coprinopsis cinerea hyphal growth, 388 allelic mutants were generated. All of these showed cross-resistance among deGlc, GA and L-sorbose (Moore & Stewart, 1971b; Moore, 1973). More importantly this research led to the discovery of two transport systems for hexose sugars : an allosteric ATP-binding cassette hexose transporter, which:
- couples hydrolysis of adenosine triphosphate (ATP) to the translocation of hexose across the hyphal membrane in the high-affinity configuration, and
- enables hexose transport by facilitated diffusion over a proton gradient in its low affinity configuration.
None of the hundreds of mutants obtained in this study could utilise fructose as a sole source of carbon due to a defect in sugar transport, though all had approximately normal levels of activity of enzymes involved in intracellular sugar metabolism. The gene symbol ftr, signifying fructose transport, was assigned to the gene because the loss of fructose transport across the membrane, from the extracellular environment to the cytoplasm, was the most easily demonstrated phenotype. Subsequent detailed kinetic analysis of sugar transport in the wild type showed that the ftr gene-product is a complex allosteric transport protein which is alone responsible for the two hexose transport systems to which reference is made above.
Using current terminology, the ftr gene product is predicted by the kinetic data obtained to be an allosteric ATP-binding cassette hexose transporter, which (a) couples hydrolysis of adenosine triphosphate (ATP) to the translocation of hexose across the hyphal membrane in the high-affinity configuration, which it assumes when sugar is in low supply; and (b) facilitates hexose transport as a glucose-proton symport by facilitated diffusion over a proton gradient in its low affinity configuration, which it assumes when extracellular glucose is abundant. More detail about the mechanisms of sugar transport in Coprinopsis cinerea can be found in this (free) PDF: Moore & Devadatham, 1979. |
Such carrier proteins are generally integral membrane proteins; meaning that they exist within, and span, the membrane across which they transport their substrates.
All these transporters have α-helical structures in their membrane-spanning domains that contribute to substrate translocation across the membrane and it is tempting to suggest that the mutant clusters observed in the ftr gene map correspond to the membrane-spanning domains of the ftr gene product, as regions of these would be responsible for, or at least take part in, the molecule-specific substrate binding. Such an interpretation would explain why:
- the kinetic characters of ftr mutants show they were defective in Vmax rather than Km for sugar uptake (that is, in rate of translocation rather than affinity);
- ftr mutants selected for resistance to different inhibitory sugar analogues mapped consistently to different positions within the gene.
Copyright © David Moore & Lily Novak-Frazer 2016