David Moore's account of his research on carbohydrate metabolism in the 1960s and 1970s
David Moore has reviewed his research career in the book ‘Coprinopsis: an Autobiography’ (Moore, 2013b)[view Amazon page], but he describes his researches in those distant days at the start of his research career, now coming up to 50 years ago, in what follows.
My Ph. D. research dealt with the biochemical genetics of Coprinus cinereus (then called Coprinus lagopus, now called Coprinopsis cinerea). This research was done in the mid-1960s, in the days of biochemical markers and auxotrophic mutants, and was a study of Mendelian ‘formal’ genetics, aimed at extending the chromosomal linkage map. Consequently, the work was highly numerate and involved extensive intercrossing, progeny counting, linkage calculations and data interpretation (Moore, 1967a), as well as purely chemical approaches to identification of precursors and intermediates in metabolic pathways and biochemical and enzymological analyses (Moore, 1967b).
When I took up my first job as Assistant Lecturer in the University of Manchester I decided to initiate a study of sugar metabolism. I made a start by determining the spectrum of carbon and energy sources that could be used by Coprinopsis cinerea (as Coprinus lagopus) (Moore, 1969) and then went on to examine the effects of a number of sugar analogues on growth of the organism.
The then-existing literature suggested that sugar analogues usually cause inhibitions to the growth of fungi by being used in metabolism in place of glucose. For example, hexokinase phosphorylates the glucose analogue 2-deoxy-D-glucose (deGlc), and its 6-phosphate inhibits the activity of other glycolytic enzymes, especially phosphohexose isomerase and glucose 6-phosphate dehydrogenase. Formation of deGlc-6P is the usual limit of metabolism of this sugar analogue and accumulation of the phosphate ester leads to a considerable drain on phosphate pools. ATP levels decline drastically and deGlc rapidly initiates degradation of purine nucleotides. In most fungi, polysaccharide synthesis is affected; preformed wall material being eroded and synthesis of new wall components prevented by sequestering of uridine and guanosine nucleotides through futile reaction with deGlc.
Another sugar analogue, L-sorbose is not phosphorylated, and the biochemical basis of the inhibitions caused by this analogue is more obscure. Some enzymes involved in polysaccharide synthesis are sensitive to inhibition by sorbose, and a characteristic of growth on this sugar is formation of an abnormally thick cell wall. Filamentous fungi grown on solid medium containing sorbose assume an abnormal growth form in which cells are much shortened and branching frequency is increased. There are indications that sorbose, perhaps by interfering with cell wall structure, affects hyphal morphogenesis and that this effect may be magnified into a macroscopic change in the characteristics of the colony during growth on agar medium. The massive change in hyphal and colony morphology which is so caused is why sorbose and related compounds are called paramorphogens.
I found that the glucose analogue 2-deoxy-D-glucose seriously inhibited the growth of Coprinopsis cinerea, and there were some correlated morphological changes (suggesting involvement of the sugar in cell wall metabolism). For example, under the influence of 2-deoxy-D-glucose, outgrowths destined to become clamp connections in Coprinopsis dikaryons continue to grow away from the parent hypha and became established as monokaryotic branches (Moore & Stewart, 1971a).
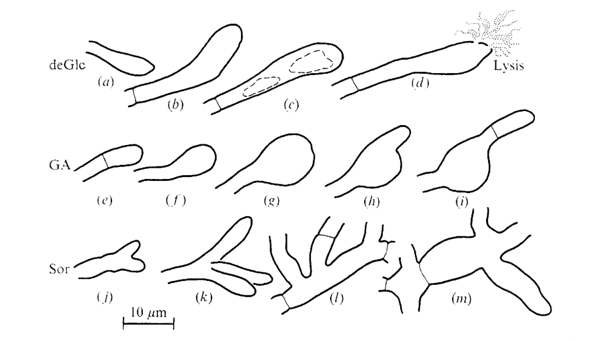
The morphologies of Coprinopsis cinerea hyphae grown on media containing inhibitory concentrations of the hexose analogues 2-deoxy-D-glucose (deGlc), D-glucosamine (GA), and L-sorbose (Sor) were illustrated by Moore & Stewart (1972) as shown in the figure below. In summary: vacuolation and eventual lysis of swollen terminal cells occurred on media containing deGlc; there was extreme swelling of terminal compartments on GA (with frequent regrowth of hyphal tips from subterminal positions); and very frequent branching and giant mycelial cells were seen on medium containing Sor. This paper (Moore & Stewart, 1972) concludes with the sentence: ‘The implication is that the processes which control the shape of the [hyphal] compartment must be very closely related to the processes which determine the chemical composition of the hyphal wall.’
|
Shapes assumed by terminal and intercalary compartments of hyphae of Coprinopsis cinerea grown on media containing inhibitory concentrations of hexose analogues. Drawings are tracings from photographs of individual hyphal compartments observed with oil immersion microscope objectives. The figures are arranged in a likely developmental series but individual hyphae were not followed through the development of these aberrations. Note the vacuolation and eventual lysis of swollen terminal cells which occurred on deGlc; the extreme swelling of terminal compartment on GA; and the frequent branching and giant mycelial cells seen on Sor. The following media were used: (a), (b), (d), 5 mM Glucose + 20 mM-deGlc; (c), 5 mM-Acetate + 0.1 mM-deGlc; (e), 5 mM Glucose + 15 mM GA; (f), (h), 5 mM Fructose + 0.2 mM GA; (g), (i), 40 mM Glucose + 80 mM GA; (j), (m), 5 mM Acetate + 2 mM Sor: (k), (I), 5 mM Glucose + 75 mM Sor. Figure and legend from Moore & Stewart, 1972. |
At this time, of course, I was employed as a Lecturer in Genetics, so having demonstrated that Coprinopsis cinerea was sensitive to hyphal growth inhibition by these sugar analogues, the obvious next step for the project was to select mutants of the organism that were resistant to these growth inhibitions.
This I did and subsequently isolated many hundreds of independently-isolated mutants of Coprinopsis cinerea which were resistant to growth inhibition by analogues of sugars. 388 mutants resistant to deGlc were isolated and shown to be allelic (that is, they were mutants in the same gene).
The mutants were pleiotropic in the sense that although they were initially selected only for resistance to 2-deoxy-D-glucose they were found to be cross-resistant to both of the related analogues, L-sorbose and D-glucosamine (Moore & Stewart, 1971b). Indeed, when mutants were later selected for resistance to L-sorbose, they were found to be cross-resistant to inhibition by deGlc and GA; and those selected for resistance to glucosamine were cross-resistant to deGlc and Sor; and all the mutants were alleles of the same gene (Moore, 1973).
Furthermore, none of these mutants were able to utilise fructose as a sole source of carbon, and we demonstrated that the inability to utilise fructose resulted from a defect in sugar transport. The gene symbol ftr was assigned to the gene, signifying fructose transport, because the loss of ability to transport fructose across the membrane from the extracellular environment to the cytoplasm was the most easily demonstrated phenotype.
However, subsequent detailed kinetic analysis of sugar transport in the wild type showed that the ftr gene-product is a complex allosteric transport protein.
Two transport systems for glucose were detected in the wild type: a high affinity system with a Km of 27 μM, and a low affinity system with a Km of 7.5 mM. Both systems accumulated against concentration gradients and both were sensitive to inhibition by metabolic poisons. Only the low affinity system could initially be demonstrated in glucose-grown tissue, although the high affinity system was restored by starvation in glucose-free medium (for example, a medium containing acetate as sole carbon source). The half-time for restoration of high affinity activity was 3.5 min and the process was unaffected by cycloheximide (which inhibits protein synthesis); indicating that the change from low to high affinity only requires a change in protein conformation.
Addition of glucose to an acetate-grown culture inactivated the high affinity system with a half-life of 5 to 7.5 seconds; again indicating change in protein conformation.
Addition of cycloheximide to an acetate-grown culture caused decay of the high affinity system with a half-life of 80 min, showing that maintenance of the high affinity system requires protein synthesis and its loss requires protein turnover.
Regulation is thus thought to depend on modulation of protein activity rather than synthesis, and the kinetics of glucose, 2-deoxy-D-glucose and 3-O-methylglucose uptake would be consistent with there being a single carrier showing negative co-operativity (Moore & Devadatham, 1979). In functional terms this means that:
- in the presence of substrate glucose the glucose transporter binds sugar molecules and takes on a shape that makes it into a low affinity, proton gradient-driven transporter;
- as external glucose levels decline the sugar molecules leave their binding sites on the transporter protein, which takes on a shape that makes it a high affinity, ATP-driven transporter that is able to scavenge for substrate to transport.
Analysis of the ftr transport defective mutants revealed defects in both transport systems although the mutants used were alleles of a single gene. So, the conclusion is that this ftr cistron is the structural gene for an allosteric molecule which serves both transport systems.
It was eventually demonstrated that ftr alleles had approximately normal levels of activity of enzymes involved in intracellular sugar metabolism, though the mutants showed greatly depressed rates of sugar uptake.
Uptake rates from 0.01 mM solutions of 2-deoxy-D-glucose were only 1 to 4% of the wild type rate, and from 15 mM solutions the mutant rates were between 16 and 40% of normal.
Kinetic analysis showed that the mutant Vmax values were reduced to a few percent of normal while Km values were relatively little changed and in some cases the mutants had an increased affinity for the substrate. Reverse mutations restored the Vmax value and the Km to about the wild type level.
Previous data had shown that the positions of inhibitor-resistant mutations within the allele map depended on the identity of the inhibitor originally used to select the mutant strain, which implied molecule-specific interaction between the ftr gene product and the inhibitory substrate (Moore & Devadatham, 1975).
Since the mutants are defective in transport from both high and low sugar concentrations, and since they exhibit coordinated alterations in Km and Vmax, it was concluded that the ftr cistron is the structural gene for a product involved in sugar translocation (both as carrier and energisation link) in both high and low affinity glucose transport systems (Taj Aldeen & Moore, 1982).
Using current terminology, the ftr gene product is predicted by these kinetic data to be an allosteric ATP-binding cassette hexose transporter, which couples hydrolysis of adenosine triphosphate (ATP) to the translocation of hexose across the hyphal membrane in one (the high-affinity) configuration and facilitates hexose transport by facilitated diffusion over a proton gradient in its low affinity configuration.
Such carrier proteins are generally integral membrane proteins; meaning that they exist within, and span, the membrane across which they transport their substrates. All these transporters have α-helical structures in their membrane-spanning domains that contribute to substrate translocation across the membrane and it is tempting (particularly because ftr mutants were defective in Vmax rather than Km) to suggest that the allele mutant clusters observed in the ftr allele map correspond to the membrane-spanning domains of the ftr gene product, as regions of these would be responsible for, or at least take part in, the molecule-specific substrate binding.
Copyright © David Moore 2016