3.4 Blastocladiomycota
Members of this phylum, which you will find called Blastocladiales in older textbooks, are saprotrophs as well as parasites of fungi, algae, plants and invertebrates, and may be facultatively anaerobic in oxygen-depleted environments. The saprotrophs are easily found on decaying fruits and plant litter. All members of this phylum have zoospores with a distinct ribosome-filled cap around the nucleus (James et al., 2014).
The thallus may be monocentric or polycentric and becomes mycelial in Allomyces. Other representative genera are: Physoderma, Blastocladiella, and Coelomomyces. Physoderma spp. are parasitic on higher plants, Coelomomyces is an obligate endoparasite of insects with alternating sporangia and gametangia stages in mosquito larvae and copepod (fish lice) hosts, respectively.
Characteristically, the Blastocladiomycota have life cycles with what is described as a sporic meiosis; that is, meiosis results in the production of haploid spores that can develop directly into a new, but now haploid, individual. This results in a regular alternation of generations between haploid gametothallus and diploid sporothallus individuals. In general terms, a multicellular diploid adult organism (the sporothallus) produces a sporangium within which meiosis occurs. Meiosis typically produces four haploid meiotic products, which are zoospores. Under proper conditions these germinate and develop into a multicellular haploid gametothallus organism. This differentiates gametangia that produce gametes by mitosis. Gametangia and gametes are both haploid. Gametes find each other, unite, and produce a diploid zygote that matures into a young diploid sporothallus to complete the life history (Fig. 2).
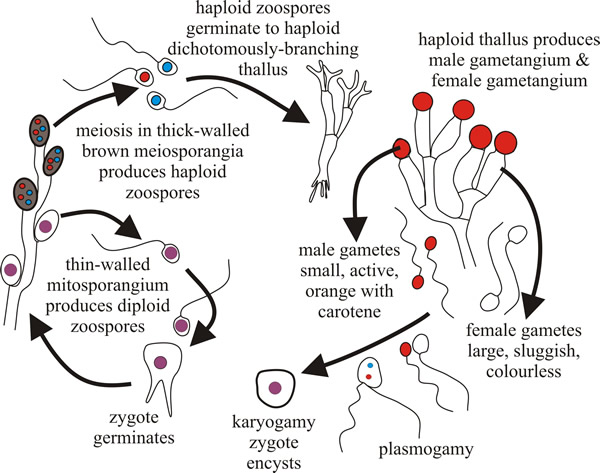 |
| Fig. 2. The Allomyces life cycle. Allomyces produces a branched thallus with marked polarity (basal rhizoids and apical sporangia). Sexual reproduction by anisoplanogametes (female gametes are colourless (produced in oogonia) and twice the size of the orange male gametes (produced in antheridia). Two different thalli are formed: haploid gametothallus and diploid sporothallus (the diploid phase is prolonged). |
Allomyces is anisogamous; female gametes are colourless and sluggish, male gametes are orange (they contain α-carotene) and very active, swimming in arcs interspersed with a jerky, tumbling, movement. This activity is an aspect of the mechanism that allows male gametes to find female gametes, which they do because female gametes produce a chemical attractant.
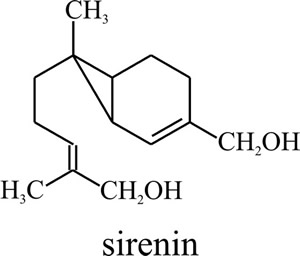 |
Fig. 3. Molecular
structure of the Allomyces male-attracting pheromone sirenin.
Biological activity requires a terminal hydroxymethyl group on the side
chain and a hydrophobic group must be present at the other end of the
sirenin molecule (Pommerville et al., 1990). The chemical gets
its name from the Sirens of Greek mythology. |
This is a hormone, called sirenin (Fig. 3), which is a sesquiterpene that consists of a cyclopropyl ring attached to an isohexenyl side chain (C15H24O2 with a molecular mass of 236).
To synthesise this molecule the female gamete converts acetyl-CoA to farnesyl pyrophosphate, which in turn is converted to sirenin. So much acetyl-CoA is diverted to form sirenin that there is diverted to form sirenin that there is less ATP available in the mitochondrion for flagella motion, which is why the female gametes are not active.
However, the male gametes are active and they swim in arcs; they have membrane receptors that respond to sirenin concentration. Sirenin stimulates the influx of calcium ions (Ca2+) into the sperm cytoplasm and the physiological response is to reduce the length of the arc in the swimming of the male gametes; that is, the pheromone influences the frequency of directional changes and the duration of the chemotactic run, the end-product being movement towards the source of the pheromone (the female).
As the male gamete nears the highest concentration of sirenin, the arcs disappear, and the tumbling motion becomes exaggerated. Thus, the male gametes are very erratic and active near the female gametes and this response ensures syngamy. The resultant zygote is a motile zoospore (with tinsel-type flagellum) that settles down in the environment to grow into a diploid thallus.
Sirenin is, therefore, a sex pheromone (a hormone produced by one partner to elicit a sexual response in the other). Only male gametes respond to sirenin, to which they are highly sensitive (sensitivity threshold about 1 × 10-10 M). In fact their sensitivity of response to sirenin (they react to as little as 20 pg ml-1) is twenty million times greater than their response to nutrients (400 µg ml-1)[pg = 10-12 g, µg = 10-6 g]. The importance of this very sensitive hormonal system in Allomyces is that it enables gametes to find each other in an aquatic ecosystem (preventing gamete loss or wastage) and by so doing increases the chance of successful sexual reproduction.
Besides sirenin, the sperm cells of Allomyces macrogynus produce a female attractant, called parisin. Some general features of this molecule suggest it may be similar structurally to sirenin in being a terpene, but the molecular nature of parisin and its effect on female gametes have not been completely resolved.
We bring this story to your attention now to make an important point about the general biology of fungi, which is that even these ‘primitive’ organisms have evolved a precise and efficient cell targeting system. This clearly comprises a very specific chemical attractant produced by one cell and a very exact receptor of that hormone in the other cell, which is linked to an intracellular signalling cascade that amplifies the signal to an extent that makes the reception process exquisitely sensitive to the hormone. We recommend our Resources Box for a little further discussion of pheromones in fungi. The point we want you to note from this discussion is that fungi produce a full chemical spectrum of hormones: terpenoid, sterol and peptide hormones. Just like animals.
Resources Box Pheromones in fungi For an outline discussion of pheromones used in mating in fungi CLICK HERE. |
Another example that reveals an important truth about fungal biology is found in the way organisms like Blastocladiella make their zoospores. Blastocladiella has been used for extensive research on reproductive physiology, biochemistry and cell biology, and use of the electron-microscope to examine the ultrastructure of zoospore formation revealed a unique feature of fungal cell biology.
To emphasise the significance of this, let’s carry out the thought experiment of working out what would happen if these fungi were either animals or plants. The situation is that we are converting the chytrid thallus, a single sac-like cell, into a sporangium. Initially there is a single nucleus, but this will undergo several mitotic divisions so that the volume of the sporangium can be subdivided into many zoospores, each of which will have a single mitotically-produced nucleus. How will that subdivision be managed?
If Blastocladiella was an animal, then at each division the dividing cell would become constricted at the equator of the mitotic spindle and two daughter cells would be produced as a result of the cleavage of the mother cell. Through successive rounds of mitosis, more and more cells would be produced; just like a developing animal embryo.
If Blastocladiella was a plant, then at each nuclear division a daughter cell wall would be formed across the equator of the mitotic division spindle. Daughter cells would then be successively halved in size (but doubled in number) as each round of mitosis occurred.
But Blastocladiella is neither animal nor plant, and it does neither of these things. Instead, Blastocladiella uses a uniquely fungal mechanism. Its zoospores are formed by cleavage of the multinucleate protoplasm in the zoosporangium, yes, but this happens as masses of cytoplasmic vesicles fuse to one another to create the borders between adjacent zoospores. We can do no better than quote the original description:
“Soon after the beginning of flagella formation it is possible to find early stages of ‘cleavage furrow’ formation ...This process … involves the fusion of many small vesicles … cleavage vesicle fusion results in progressive expansion of the primary cleavage furrows and it appears that this activity is simultaneously initiated at many points. Occasionally vesicles can be found in somewhat linear arrangements over a short distance. They more often occur in less orderly clusters and fuse in irregular ring-shaped patterns lying roughly in the plane of the developing cleavage furrow. The frequent occurrence of cytoplasmic peninsulas surrounded by U-shaped areas of cleavage vesicle suggests that many of the rings may in fact be short cylinders; if so, the closure and interconnection of the rings may be irregular and only gradually assume the form of a regular furrow. … The cleavage furrows also fuse with the earlier formed vesicles surrounding the flagella with the result that these finally lie within the cleavage furrows and outside of the uninucleate blocks of cytoplasm delineated by the [newly formed] membrane system” (Lessie & Lovett, 1968; CLICK HERE to see the illustration of these events).
This remarkably precise zoospore generating pattern is repeated throughout the chytrids, and indeed throughout the fungi. Compare the description quoted above with this description of sporogenesis in the mucoraceous (terrestrial) fungus Gilbertella persicaria (Bracker, 1968):
“…During cleavage, the principal structural changes involve pattern transformations of protoplasmic membranes... small vesicles are formed, apparently from special cisternae [of the endoplasmic reticulum]. The disappearance of these initial vesicles coincides with the appearance of cleavage vesicles … distinguished by the presence of granules on the inner surface of the vesicle membrane ... Cleavage is initiated endogenously by the coalescence of cleavage vesicles to form a ramifying tubular cleavage apparatus. The cleavage apparatus demarcates the boundaries of potential spore initials. Lateral expansion of elements of the cleavage apparatus results in furrow-like configurations which converge to cut out spore initials as independent cells. The cleavage membrane is transformed to the plasma membrane of spore initials during late cleavage … The marker granules that were present around the periphery of the cleavage vesicles are found on the outer surfaces of spore plasma membranes after cleavage. The granules fuse to form a continuous spore envelope, and subsequently the spore wall is laid down centripetally. Thus, the envelope becomes the outermost spore wall layer…”
The process described here has been called ‘free cell formation’ and for ascospore formation has also been summarised in a similar way (Reeves, 1967):
“… A summary of the main points of free cell-formation is as follows: 1. in the 8-nucleate ascus each of the haploid nuclei forms a beak with a persistent central-body and astral rays at the tip of the beak; 2. the astral rays swing outward and down and form a thin membrane which cuts out the young spore; 3. the membrane around each spore separates the sporoplasm and included nucleus, leaving the epiplasm in the ascus…”
We are emphasising this point because it makes the general rule that where a volume of cytoplasm needs to be subdivided in fungi, the mechanism depends on the organised distribution of cytoplasmic microvesicles; the microvesicles then fuse together to create the separation of the cytoplasm. This is the way the fungi do it (and a similar cleavage system produces zoospores in sporangia of the fungus-like Oomycota), so note well this major difference from plants (no cross-walls formed) and animals (there is no constrictive cell cleavage).
We find this mechanism to be remarkable and worthy of emphasis because it raises so many questions about the molecular mechanism(s) involved in determining how the cytoplasmic domains contributing to each individual spore are defined. We have chosen to illustrate the point with quotations from papers published at about the same time in the late 1960s to illustrate another point that we find remarkable: which is that we can’t describe the mechanism(s) in much more detail 50 years later, as this quotation reveals:
“…Free cell formation is generally considered a specific feature of the Ascomycotina although it is evidently shared or partly shared with the Basidiomycotina. Early stages of basidiospore development follow the same general pattern as that of the free cell formation process in the Ascomycotina: the haploid nuclei become free in the cytoplasm and develop into individual cells together with part of the plasma from the mother cell … The Basidiomycotina are specialised by way of their nuclei and part of the plasma, which are forced to migrate by a vacuolation process, through a sterigma into a special structure formed by the sporangium wall, which will be cut off from the basidium and in which the spore formation is completed.” (Tehler et al., 2003).
It’s a pity that the molecular mechanism(s) involved in determining such a crucial aspect of the unique cell biology of fungi is/are still unknown. It's also a golden opportunity for future research.
Updated July, 2019
