The problem
It has been estimated that there are 37 billion kg of hazardous organopollutants produced annually in the USA alone, of which only 10% are safely disposed of. Organopollutants are polluting carbon-containing chemicals that are used in industry, technology and in the home. Many of them are pesticides, and while we know about DDT (an insecticide that is now banned worldwide) and creosote (banned for domestic use in the UK). But whilst these are household names, we do not know about the other everyday chemicals that contaminate our environment and endanger every organism within it. The current regulatory system allows synthetic chemicals to be used unless they are proved beyond doubt to be dangerous. Therefore until proved harmful, a chemical is deemed safe.
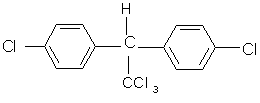
This has contributed to a persistent mistreatment of our environment and also has resulted in the effluent and waste from industrial processes making many land areas toxic to organisms. The consequences of the long persistence times in the environment of these chemicals are not yet fully known, but we are already seeing the effects of one of the most dangerous chemicals that has been in use, namely DDT (= 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane)(Fig. 1).
DDT was widely used to kill mosquitoes carrying the malarial parasite until the 1970s and was estimated by the World Health Organization (WHO) to have saved 25 million lives. However it cannot be metabolised easily, and as a result builds up in the fatty tissues of animals. Of high toxicity to fish, it is also passed along the food chain up to higher trophic levels, and by that means is a risk to humans (Fig. 2).
 |
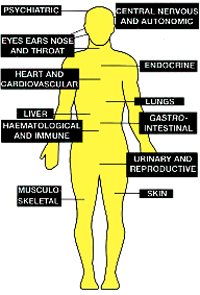 |
| Fig. 1. Chemical structure of DDT. | Fig. 2. Where DDT can damage the human body. |
The risks to humans posed by DDT are unpleasant (Fig. 2): cancer of all kinds, birth deformations, miscarriages, cardiovascular problems, interference with the central nervous system and many more.
All the organopollutants are xenobiotic compounds, meaning that they are artificially produced, and would never be seen naturally in the environment. The next section gives an overview of the organopollutants in summary form, not a comprehensive list by any means.
The major organopollutants
PAHs (polycyclic aromatic hydrocarbons): these are the most common of the organopollutants, being formed from many different sources, such as fossil fuel burning, natural oil deposits, vegetation decomposition, transport, industrial processes. Major constituents of creosote, they are usually formed from the burning of organic material. They are one of the constituents of tobacco smoke. Some chemical names that might be familiar are: benzopyrene, anthracene, phenanthrene and naphthalene. They are all very toxic and persistent, they are carcinogenic and some metabolites can bind to DNA and form mutations, thus making them mutagenic.
PCBs (polychlorinated biphenyls): also used in pesticides, they have strong thermal and electrical properties that makes them ideal for use in industrial applications like flame retardants, solvents, insulators, and in the textile and printing industries. Marketed under the names of Aroclor, Declor and more.
Pesticides (herbicides/insecticides/fungicides): these include the organochlorines: DDT, aldrin, lindane, PCP, endosulfan, and organophosphates.
-
DDT: see above;
-
aldrin: an organochlorine insecticide widely used until the 1970s, belongs to a family of chemicals that includes the insecticides dieldrin and heptachlor, all of which are strongly persistent in soil.
-
Lindane (γ-isomer of 1,2,3,4,5,6-hexachlorocylohexane): used for many years both as an agricultural insecticide and as a pharmaceutical treatment for lice and scabies. Its use for broadcast use in agriculture is now being restricted by international agreements (in 2009 the production and agricultural use of lindane was banned under the Stockholm Convention on persistent organic pollutants). However, it continues in use to control lice and scabies, and it is the foremost pesticide used in cocoa plantations, raising fears about desert chocolate being contaminated with it (view http://green.yahoo.com/blog/care2/82/chocolate-could-contain-pesticides.html).
-
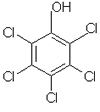
PCP (pentachlorophenol): used in agriculture and as wood preservative amongst other uses, a priority pollutant according to the US Environmental Protection Agency (EPA) because it is so strongly chlorinated (Fig. 3).
-
Endosulfan: has been used extensively around the world as an efficient agricultural pesticide but has been linked with human health problems and may be an endocrine disruptor. The international agreement to ban its use was not completed until April 2011, largely because of the refusal of India and China to agree, and there are still 14 exempted crops in Indian agriculture for a 5 year phase-out period (view http://www.rsc.org/chemistryworld/News/2011/May/06051102.asp).
-
Organophosphates: organophosphate is a general term for any ester of phosphoric acid. Strictly speaking, DNA and RNA (and other nucleotides) are organophosphates, but the organophosphates that cause concern are those with a direct C-P bond, like the nerve gases sarin and VX and those that are highly effective insecticides like parathion (O,O-Diethyl O-(4-nitrophenyl) phosphorothioate) and malathion (2-(dimethoxyphosphinothioylthio) butanedioic acid diethyl ester). Organophosphate pesticides degrade rapidly by hydrolysis on exposure to sunlight, air, and soil, so they are generally not as persistent in the environment as organochlorines, but they pose significant acute and chronic health risks in humans exposed to the agents because they all inhibit acetylcholinesterase (responsible for hydrolysis of the neurotransmitter acetylcholine).
 |
 |
| Fig. 3. Chemical structure of PCP. | Fig. 4. Fruit trees being sprayed with pesticide in Italy. |
Other contributors to organopollution are:
- Munitions like TNT (trinitrotoluene) which pollutes military training areas as well as war zones and can be persistent pollutants of local soil and water supplies.
- Bleach plant effluents: traditional paper pulp bleaching requires a chlorine-mediated process and the effluent from these pulp mills will contain chlorophenols produced by reaction of the bleach with phenolics from lignin.
- Synthetic dyes: like azo-dyes (which characteristically feature two nitrogen atoms linked with a double bond), anthraquinone, triarylmethane, all of which produce intense colours and are used in textile dyeing, paper printing, colour photography and petroleum products, though it has been estimated that between 10 and 15% of the dyes used end up in the manufacturing effluent, and are in danger of contaminating the local environment. Azo-dyes are the predominant dyes used in industry, being responsible for around half of all dyeing processes. They are recalcitrant in water (meaning they resist biodegradation) and hence are recalcitrant when absorbed into the local environment from the factory effluent. Many, like the acridines, are also carcinogenic.
Processes used to clear some of these persistent pollutants
Of course there are many methods already in practice that attempt to rid the environment, or at least reduce the amount in the environment, of these poisonous chemicals, but they are neither cost effective (estimated cost of US$1 TRILLION to decontaminate toxic waste sites in USA using traditional methods) nor efficient. Whilst some of these chemicals can be degraded by microorganism in soil like bacteria, the process is usually slow, which results in persistence, and the metabolic products are often as toxic as the primary polluting substance.
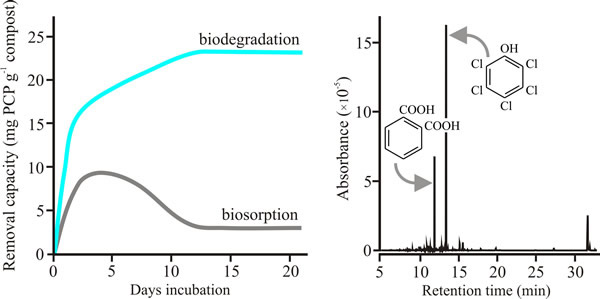
What these organopollutants have in common, apart from being recalcitrant chemicals, is that many of them have strong similarities to lignin; typically, by being aromatic compounds (containing a benzene ring). And this is where the white rot fungi step into play. By being the only microorganisms capable of lignin degradation, they have enormous potential in the degradation of these organopollutants. An example of what is possible is shown in Fig. 5.
The conventional remediation strategy for PCP contaminated land is excavation and incineration or landfilling. Such methods are expensive, obviously destructive to the environment and ineffective for anything other than highly localised point source pollution. Bioremediation is a very promising alternative, using biological systems for the environmental clean up. The ability of a range of known wood-decay or plant-litter-decay fungi to remove PCP from a batch culture was compared. Fungi tested as mycelia were: Armillaria gallica, A. mellea, Ganoderma lucidum, Lentinula edodes, Phanerochaete chrysosporium, Pleurotus pulmonarius, a Polyporus species, Coprinopsis cinerea and Volvariella volvacea, and the spent mushroom compost from farm beds growing the oyster mushroom Pleurotus pulmonarius was also tested. All these fungi showed active breakdown and absorption of PCP removal mechanisms, though the tolerance level of the fungus towards PCP did not correlate with its degradative capacity. In a 7-day incubation, Armillaria mellea mycelium showed the highest degradative capacity (13 mg PCP g-1 mycelium dry weight) and Pleurotus pulmonarius mycelium was second highest with 10 mg PCP g-1 mycelium dry weight; the least effective was Polyporus with 1.5 mg PCP g-1 mycelium dry weight. On the other hand, the Pleurotus spent mushroom compost, harbouring both bacteria and fungi, had a degradative capacity of 19 mg PCP -1 dry weight in only 3 days exposure to PCP (Fig. 5)(Chiu et al., 1998; download PDF).
 |
|---|
| Fig. 5. Data showing that incubation for a few weeks with spent Oyster Mushroom compost leads to destruction of pentachlorophenol (not just its adsorption)(left hand panel), and that mycelium of Pleurotus pulmonarius dechlorinates pentachlorophenol (PCP) through a catabolic process that involves removal of the chlorine followed by opening of the benzene ring (right-hand panel). For the left-hand plot, absolute removal capacity of PCP by spent oyster mushroom substrate (that is, the substrate left after the last crop was harvested) was quantified by capillary electrophoresis. The right-hand panel shows a mass-selective gas chromatography (GC-MS) spectrum of the extract of the fungal biomass of Pleurotus pulmonarius mycelium after 2 days incubation in a medium containing 25 mg l-1 pentachlorophenol. The most prominent peak (retention time 13.53 min) is PCP, the next most prominent peak at 12.03 min is benzene-1,2-dicarboxylic acid (also called phthalic acid). Other peaks at longer retention times include fatty acids that result from opening the benzene ring and esterification of its straight-chain derivatives. For example, peaks at retention times of 15.73, 31.96 and 32.25 min have been identified as hexadecanoic acid (palmitic acid, C16H32O2) and its derivatives. Figure and caption from Moore et al., 2011 (URL). Data from Chiu et al., 1998 (download PDF). |
Mass-selective gas chromatography (GC-MS) chromatograms revealed only residual PCP peaks in extracts of PCP-treated spent mushroom substrate extracts, a contrast with the mycelial incubations in which a variety of breakdown products were detectable. The spent compost left after oyster mushroom cultivation does two crucial things. It absorbs, immobilizes and concentrates PCP so it can be transported away from the contaminated site, and it also digests PCP completely. Mushroom cultivation is a common practice all over the world and the idea that hazardous waste materials could have their pollutants removed and produce a mushroom crop at the same time is exceedingly attractive.
But the idea is not free of problems. Oyster mushrooms can concentrate the metal cadmium (a common industrial contaminant) to such an extent that by eating less than 30 g (dry weight) of the most contaminated samples you would exceed the weekly limit tolerated by humans. Cadmium is so toxic that this situation could pose a public health hazard. There are no worries about conventionally cultivated oyster mushrooms. The point is that if the mushroom is grown on composts that might be mixed with industrial wastes (in remediation programs, for example), then it would be advisable to monitor the heavy metal contents before mushrooms are marketed for food. By far the most promising technique is use of the spent mushroom substrates remaining after harvesting mushroom crops. Ironically, these are often discarded as wastes themselves, but they are clearly able to offer an integrated approach combining soil conditioning with degradation of pollutants as an effective strategy for bioremediation in situ.
Further information about bioremediation can be found in the new textbook 21st Century Guidebook to Fungi by David Moore, Geoffrey D. Robson & Anthony P.J. Trinci. Published 2011 by Cambridge University Press: ISBN: 9780521186957. URL: ttp://www.cambridge.org/gb/knowledge/isbn/item6026594/?site_locale=en_GB. View Amazon page.
Updated December 15, 2016
