Rhythms, oscillators and clock genes |
|||||||
Circadian rhythms are biological rhythms with periods of about 24 hours. Rhythmicity is endogenous and self-sustaining when environmental conditions are constant; the length of the rhythmic cycle being genetically determined. Rhythmically changing environmental signals, particularly of light and temperature, set the phase of the endogenous rhythm and adjust it to exactly 24 hours. Circadian rhythms are ubiquitous in eukaryotes and have also been demonstrated in cyanobacteria. It does not require complex tissue organization, and even single cells can express rhythmicity. The underlying molecular mechanism is a feedback loop involving both positive and negative elements, centred on the transcription of clock genes and translation of clock proteins. The positive element in the loop is the transcriptional activation of a clock gene(s) through binding of paired transcriptional activators on the clock gene promoter; they are paired by virtue of interaction via PAS domains (see below). Translation of the transcribed message of the clock gene (which is subject to additional regulation) generates a clock protein that is the negative element of the feedback loop. This blocks activation of the clock gene so the amount of clock gene mRNA declines and eventually the levels of clock protein also decline. This generates a daily cycle of clock gene mRNA and clock proteins, and forms an oscillator that generates what is known as an ‘output’ that is the basis of the timing signal that controls rhythmical cellular functions (which might be organism-, organ- or even cell-specific) (Fig. RB 1).
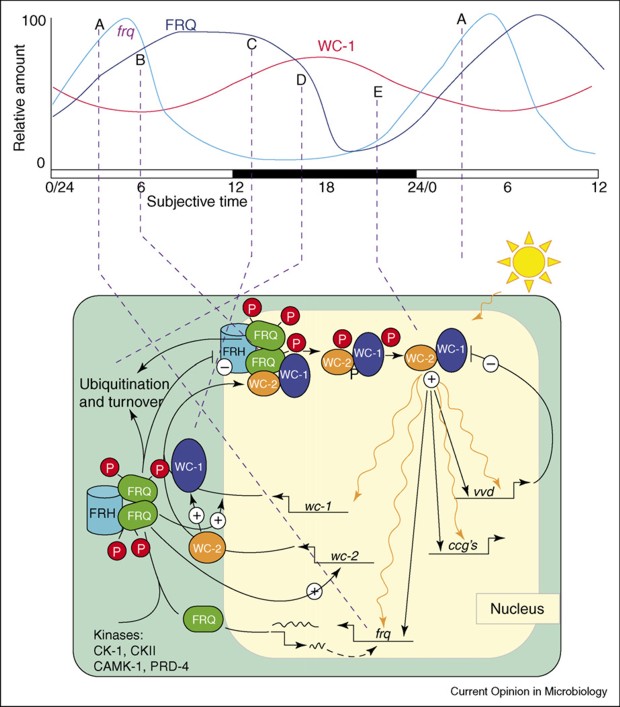
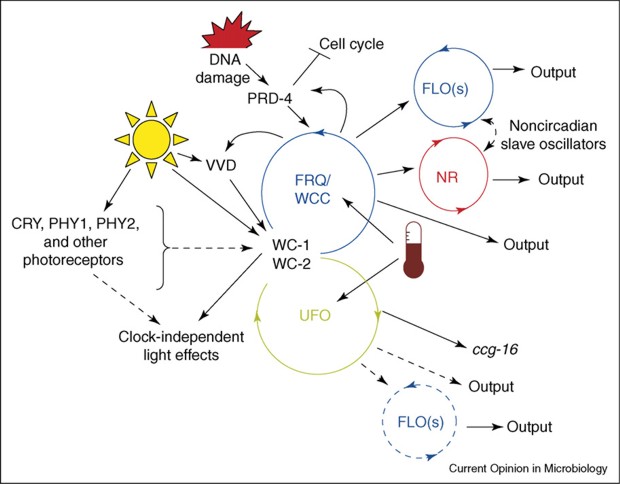
A circadian system can be made up of one or more interconnected feedback loops forming quite a complex network. In addition, the system receives inputs of ambient light and temperature to adjust its phase so that the internal day matches the external day, and then uses the time information it generates to regulate the life of the cell and of the organism as a whole. The overall pattern in Neurospora is shown in Fig. RB 2.
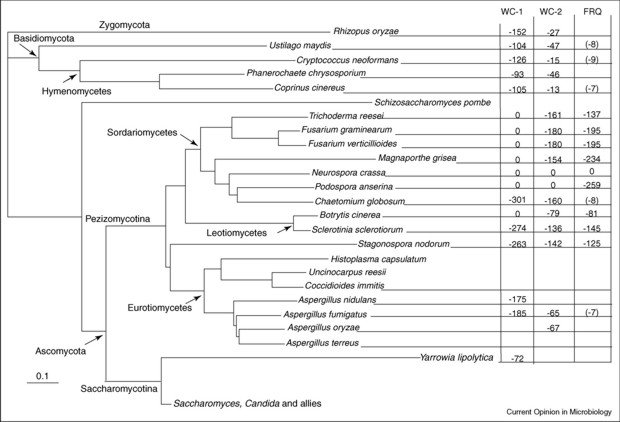
The Neurospora system involves the proteins of mutants called white collar-1 (WC-1), white collar-2 (WC-2), and frequency (FRQ)(homologues of frequency in Drosophila are period and timeless). Many clock gene proteins have a common structural motif known as the PAS domain. PAS is an acronym created from ‘Per-ARNT-Sim’ and PAS domains were first identified in the Drosophila proteins PER and ARNT and they were later found in a wide range of organisms. In circadian rhythmicity the PAS-domain proteins act as heterodimeric transcriptional activation complexes to drive expression of clock genes. Interestingly, though, PAS domains mediate protein-protein interactions in response to stimuli when cofactors bind within their hydrophobic cores, so they have important roles as sensory modules for a wide range of environmental conditions including oxygen tension, redox potential, carbon monoxide, nitric oxide, as well as light intensity and temperature, which are the main inputs to the Neurospora circadian oscillators. Light acts in Neurospora to induce transcription of the negative elements that reset the clock and synchronise the cell to the daily light/dark cycle. Temperature-influenced translational regulation of FRQ synthesis in Neurospora sets the physiological temperature limits over which the clock operates. The circadian system of Neurospora has been developed as an important model system for understanding circadian rhythms, and there is evidence for conservation of rhythmicity mechanisms in filamentous fungi (Fig RB 3).
ReferencesDunlap, J.C. (1998). Common threads in eukaryotic circadian systems. Current Opinion in Genetics & Development, 8: 400-406. CLICK HERE to download the complete text. Dunlap, J.C. & Loros, J.J. (2006). How fungi keep time: circadian system in Neurospora and other fungi. Current Opinion in Microbiology, 9: 579-587. DOI: http://dx.doi.org/10.1016/j.mib.2006.10.008. CLICK HERE to download the complete text. Lakin-Thomas, P.L. (2000). Circadian rhythms: new functions for old clock genes? Trends in Genetics, 16: 135-142. CLICK HERE to download the complete text. |
|||||||
Close the window to return to 21st Century Guidebook to Fungi
This is a Resources Box from the 21st Century Guidebook to Fungi: © David Moore, Geoffrey D. Robson and Anthony P. J. Trinci 2019